Abstract
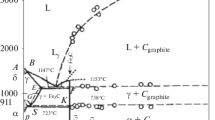
A critical review of published data provides a fairly accurate knowledge of the thermodynamic properties of all of the phases of the system Fe-C that are stable or metastable at atmospheric pressure. Selected data are shown as tables and equations. A proposed phase diagram differs only slightly from others recently published but has the following features. Peritectic compositions and the α-γ equilibrium are shown to agree with measured values of the activity of iron in the solid and liquid solutions and the thermodynamic properties of pure iron. Of all the reported carbides of iron only two may be studied under equilibrium conditions. The solubilities of cementite and of χ-carbide in α-Fe are deduced from measured equilibria. Both are metastable at all temperatures with respect to graphite and its saturated solution in iron. The χ-carbide becomes more stable than cementite below about 230° Certain published data on ε-carbide permit an estimate of its free energy as a precipitate during the aging process.
Similar content being viewed by others
References
M. Hansen and K. Anderko:Constitution of Binary Alloys, McGraw-Hill, New York, 2nd ed., 1958.
J. F. Elliott and M. Gleiser:Thermochemistry for Steelmaking, Addison-Wesley, Reading, Massachusetts, Vol. I, 1960.
M. Benz and J. F.Elliott:Trans. TMS-AIME, 1961, vol. 221, pp. 323–31;Trans. TMS-AIME, 1961, vol. 221, p. 888.
R. A. Buckley and W. Hume-Rothery:J Iron Steel Inst., a) 1960, vol. 196, pp. 403–06; b) 1962, vol. 200, pp. 142–43.
L. S. Darken and R. W. Gurry:The Physical Chemistry of Metals, McGraw-Hill, New York, 1953.
R. Hultgren, R. L. Orr, P. D. Anderson, and K. K. Kelley:Selected Values of Thermodynamic Properties of Metals and Alloys, John Wiley and Sons, New York, 1953.
E. Scheil, T. Schmidt, and J. Wünning:Arch. Eisenhuettenw., 1961, vol. 32, pp. 251–60.
S. Ban-ya, J. F. Elliott, and J. Chipman:Met. Trans., 1970, vol. 1, pp. 1313- 20.
J. C. Swartz:Trans. TMS-AIME, a) 1967, vol. 239, pp. 68–75; b) 1969, vol. 245, pp. 1083–91.
A. Ferrier and M. Olette:C. R. Acad. Sci., Paris, 1962, vol. 254, pp. 2322–24.
J. P. Morris, E. F. Foerster, C. W. Schultz, and G. R. Zellars:U. S. Bur. Mines, Rept. Invest, no. 6723, 1966.
M. Olette and A. Ferrier:The Physical Chemistry of Metallic Solutions and Intermetallic Compounds, Nat. Phys. Lab., U. K., Symp. No. 9, Paper 4H, H.M. Stationery Office, London, 1959.
P. D. Anderson and R. Hultgren:Trans. TMS-AIME, 1962, vol. 224, pp. 842- 45.
W. A. Dench and O. Kubaschewski:J. Iron Steel Inst, 1963, vol. 201, pp. 140- 43.
M. Braun: Übe die spezifische Wärme von Eisen, Kobalt, und Nickel im Bereich hoher Temperaturen, Inaugural Dissertation, Universität zu Köln, Germany, 1964.
D. C. Wallace, P. H. Sidles, G. C. Danielson:J. Appl. Phys., 1960, vol. 31, pp. 168–76.
R. L. Orr and J. Chipman:Trans. TMS-AIME, 1967, vol. 239, pp. 630–33.
F. D. Rossini:J. Chem. Thermodynamics, 1970, vol. 2, pp. 447–59.
H. Dünwald and C. Wagner:Z. Anorg. Allgem. Chem., 1931, vol. 199, pp. 321–46.
R. P. Smith:J Am. Chem. Soc., 1946, vol. 68, pp. 1163–75.
H. Schenck and H. Kaiser:Arch. Eisenhuettenw., 1960, vol. 31, pp. 227–35.
E. Schürmann, T. Schmidt, and H. Wegener:Giesserei, 1964, vol. 16, pp. 91–98.
C. Wells:Trans. ASM, 1938, vol. 26, p. 289.
R. W. Gurry:AIME Trans., 1942, vol. 150, pp. 147–53.
F. Adcock:J. Iron Steel Inst, 1937, vol. 135, p. 281.
F. D. Richardson and W. E. Dennis:Trans. Faraday Soc, 1953, vol. 49, pp. 171–80.
J. Chipman:Met Trans., 1970, vol. 1, pp. 2163–68.
T. Syu, A. V. Polyakov, and A. M. Samarin:lzv. Vyssh. Ucheb. Zaved., Chem. Met, 1959, no. 11, pp. 3–12.
R. Ruer and J. Biren:Z. Anorg. Allgem. Chem., 1920, vol. 113, pp. 98–112.
J. Chipman, R. M. Alfred, L. W. Gott, R. B. Small, D. M. Wilson, C. N. Thomson, D. L. Guernsey, and J. C. Fulton:Trans. ASM, 1952, vol. 44, pp. 1215–30.
J. A. Kitchener, J. O’M. Bockris, and D. A. Spratt:Trans. Faraday Soc, 1952, vol. 48, p. 608.
J. A. Cahill, A. D. Kirshenbaum, and A. V. Grosse:Trans. ASM, 1964, vol. 57, pp. 417–26.
A. A. Vertman, V. K. Grigorovich, N. A. Nedumov, and A. M. Samarin:Dokl. Akad. Nauk. SSSR, 1964, vol. 159, pp. 121–24.
J. C. Ruth and M. Turpin:C. R. Acad. Sci., Paris, 1967, vol. 265, pp. 786–88.
C. Boulanger:C. R. Acad. Sci., Paris, 1955, vol. 241, p. 1133.
R. F. Mehl and C. Wells:Trans. AIME, 1937, vol. 125, p. 429.
R. P. Smith and L. S. Darken:Trans. AIME, 1959, vol. 215, p. 727.
E. Schürmann, T. Schmidt, and F. Tillmann:Giessereiforschung, 1967, vol. 19, pp. 35–41.
R. P. Smith:Trans. TMS-AIME, 1962, vol. 224, pp. 105–11.
N. J. Petch:J. Iron Steel Inst., 1944, vol. 149, pp. 143–50.
W. Glud, K. V. Otto, and H. Ritter:Ber. Ges. Kohlentech., 1929, vol. 3, p. 40.
U. Hofmann and E. Groll:Z. Anorg. Allgem. Chem., 1930, vol. 191, p. 414.
H. A. Bahr and V. Jessen:Ber. Deut. Chem. Ges., 1933, vol. 66, p. 1238.
G. Hägg:Z. Kristallogr., 1934, vol. 89, p. 92.
K. H. Jack:Proc. Roy. Soc., 1948,A, vol. 195, p. 56.
L. S. Darken and R. W. Gurry:AIME Trans., 1951, pp. 1015–18.
R. P. Smith:Trans. TMS-AIME, 1959, vol. 215, pp. 954–57.
M. Hillert:Acta Met., 1955, vol. 3, pp. 37–38.
T. Watase:J. Chem. Soc. Japan, 1933, vol. 54, pp. 110–32.
L. C. Browning, T. W. DeWitt, and P. H. Emmett:J. Am. Chem. Soc, 1950, vol. 72, pp. 4211–17.
H. Seltz, H. J. McDonald, and C. Wells:AIME Trans., 1940, vol. 140, pp. 263- 78.
C. Schwarz and H. Uhlich:Arch. Eisenhuettenw., 1936, vol. 10, p. 11.
G. Naeser:Mitt. Kaiser Wilhelm Inst. Eisenforsch. Düsseldorf, 1934, vol. 16, pp. 207–10.
K. K. Kelley and E. G. King:U. S. Bur. Mines Bull. no. 592, 1961.
J. Mazur and W. Zacharko:ActaPhys. Polon., 1969, vol. 35, pp. 91–99 (in English).
S. Umino:Sci. Repts. Tohoku Imp. Univ., 1935, ser. 1, vol. 23, p. 665.
K. K. Kelley:U. S. Bur. Mines Bull. no. 584, 1960.
G. Borelius and S. Berglund:Ark. Fys., 1951, vol. 4, p. 173.
J. P. Sénateur, R. Fruchart, and A. Michel:C. R. Acad. Sci., Paris, 1962, vol. 255, p. 1615.
L. J. E. Hofer:U. S. Bur. Mines, Bull. no. 631, 1966.
0. Krisement:Ark. Fys., 1953, vol. 7, no. 27, pp. 353–55.
Mme. P. Lasage:Ann. Chim., 1961, vol. 6, no. 13, p. 623.
K. H. Jack:J. Iron Steel Inst., 1951, vol. 169, pp. 26–36.
A. L. Tsou, J. Nutting, and J. W. Menter:J. Iron Steel Inst., 1952, vol. 172, pp. 163–71.
J. Butler, P. Chollet, and C. Crussard:C. R. Acad. Sci, Paris, 1962, vol. 225, pp. 2961–63.
R. A. Arndt and A. C. Damask:Acta Met., 1964, vol. 12, p. 341.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chipman, J. Thermodynamics and phase diagram of the Fe-C system. Metall Trans 3, 55–64 (1972). https://doi.org/10.1007/BF02680585
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02680585