Abstract
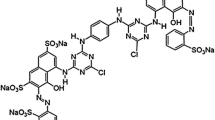
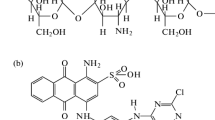
The adsorption of vinyl sulfone type reactive black 5 (RB 5) in aqueous solution onto chitosan beads and cross-linked chitosan beads with glutaraldehyde has been investigated in terms of initial pH and temperature of the solution. The adsorption equilibrium data were correlated with three adsorption models, such as Langmuir, Freundlich and Sips isotherms. Among them, the Freundlich isotherm best fit the data over the entire pH and temperature range of the solution. The adsorption capacity of RB 5 onto chitosan beads and cross-linked chitosan beads increased with decreasing initial pH and with increasing temperature. Equilibrium amount of RB 5 on chitosan beads was greater than that of cross-linked chitosan beads at the same initial pH values. Thermodynamic studies have also been carried out and values of standard free energy (°Gℴ), enthalpy (°Hℴ) and entropy (°Sℴ) were calculated.
Similar content being viewed by others
References
Bonneau, M. C.,“The Chemistry of Fabric Reactive Dyes,”J. Chem. Educ.,72, 724 (1995).
Chiou, M. S. and Li, H. Y.,“Equilibrium and Kinetic Modeling of Adsorption of Reactive Dye on Cross-linked Chitosan Beads,”J. Hazardous Materials, 2852, 1 (2002).
Choi, J. W., Song, H. K., Lee, W., Koo, K. K., Han, C. and Na, B. K., “Reduction of COD and Color of Acid and Reactive Dyestuff Wastewater Using Ozone,”Korean J. Chem. Eng.,21, 398 (2004).
Choi, B. U., Nam, G. M., Choi, D. K., Lee, B. K., Kim, S. H. and Lee, C. H., “Adsorption and Regeneration Dynamic Characteristics of Methane and Hydrogen Binary System,”Korean J. Chem. Eng.,21, 821 (2004).
Davila-Jimenez, M. M., Elizalde-Gonalez, M. P. and Pelaez-Cid, A. A., “Adsorption Interaction between Natural Adsorbents and Textile Dyes in Aqueous Solution,”Colloids and Surfaces A: Physicochem. Eng., Aspects,254, 107 (2005).
Do, D. D.,Adsorption analysis: Equilibria and Kinetics, Series on Chemical Engineering, 2, Imperial college Press (1998).
Guibal, E., Jansson-Charrier, M., Saucedo, I. and Le Cloirec, P., “Enhancement of Metal Ion Sorption Performance of Chitosan: Effect of the Structure on the Diffusion Properties,”Langmuir,11, 591 (1995).
Hsien, T.Y. and Rorrer, G. L., “Effects of Acylation and Crosslinking on the Material Properties and Cadmium Ion Adsorption Capacity of Porous Chitosan Beads,”Sep. Sci. Technol.,30, 2455 (1995).
Juang, R. S., Tseng, R. L., Wu, F. C. and Lee, S. H., “Adsorption Behavior of Reactive Dyes from Aqueous Solutions on Chitosan,”J. Chem. Tech. Biotechol.,70, 391 (1997).
Juang, R. S., Wu, F. C. and Tseng, R. L., “Use of Chemically Modified Chitosan Beads for Sorption and Enzyme Immobilization,”Advances in Environmental Research,6, 171 (2002).
Kim, T.Y., Park, S.K., Cho, S.Y., Kim, H. B., Kang, Y., Kim, S. D. and Kim, S. J., “Adsorption of Heavy Metals by Brewery Biomass,”Korean J. Chem. Eng.,22, 99 (2005).
O’Neill, C., Hawkes, F. R., Hawkes, D. L., Lourenco, N. D., Pinheiro, H.M. and Delee, W., “Colour in Textile Effluents Sources, Measurement, Discharge Consents and Simulation: A Review,”J. Chem. Technol. Biotechnol.,74, 1009 (1999).
Son, B. C., Park, K. M., Song, S. H. and Yoo, Y. J., “Selective Biosorption of Mixed Heavy Metal Ions using Polysaccharides,”Korean J. Chem. Eng.,21, 1168 (2004).
Wu, F.C., Tseng, R. L. and Juang, R. S., “Enhanced Abilities of Highly Swollen Chitosan Beads for Color Removal and Tyrosinase Immobilization,”J. Hazard. Mater.,B81, 167 (2001).
Zheng, L.Y. and Xiao, Y. L., “Penicillium sp. ZD-Z1 Chitosanase Immobilized on DEAE Cellulose by Cross-linking Reaction,”Korean J. Chem. Eng.,21, 201 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, T.Y., Cho, S.Y. Adsorption equilibria of reactive dye onto highly polyaminated porous chitosan beads. Korean J. Chem. Eng. 22, 691–696 (2005). https://doi.org/10.1007/BF02705784
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705784