Abstract
Enrichment of methanogenic cultures on methanol from the microbial population in the anaerobic digesters operated on agricultural wastes revealed a high rate of biomethanation efficiency. Routine maintenance of this enrichment in a minimal basal medium at room temperature resulted in maximal growth in 40–50 d, and indicated pigment production toward the end of the growth phase. The cultures grown in three different media, with different substrates under light and dark conditions, were analyzed for protein, pigment, and gaseous products, and morphological studies were carried out by light, phase-contrast, fluorescence, and electron microscopy. In different media with methanol as substrate, growth and pigment production were maximal for the light-grown cells, decreasing in the order: phototrophic (PS(m)) > mineral > basal medium. Methanation and phototrophic growth were inversely correlated under lightgrown conditions. In contrast, growth in the dark was predominently methanogenic in the decreasing order: mineral > basal > PS (m). Among other growth conditions tested, utilization of phototrophic substrates under light and dark conditions indicated the following:
-
1.
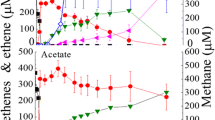
Basal and mineral media were supportive of methanogenic growth under both light and dark conditions, although methane yields under light-grown conditions were low;
-
2.
Among the different substrates tested, methanol-grown cells gave the highest methane yield in the dark and;
-
3.
Phototrophic growth in PS medium with succinate, malate, and pyruvate was better than that with methanol.
Absorption spectra of light-grown cells indicated the presence of bacteriochlorophyll a (Bchl a), as a doublet in the 800–0 nm region, which was absent in the dark-grown cells. Spectra of extracted pigments confirmed the presence of Bchl a with a 770-nm peak and carotenoid absorption bands in the 400–500 nm region indicative of the presence of a pigment of the spirilloxanthin type. Collective evidence for the predominant growth of a phototrophic organism under lightgrown conditions and microscopic examination under all conditions indicated the possible presence in the mixed culture of purple nonsulfur bacteria of theRhodopseudomonas type. In addition, the enrichment culture was found to contain other morphological forms, such as short and long rods, both individually and in clusters and coccoid cells.
The presence of such different forms of microbial population in a methylotrophic enrichment along with phototrophic bacteria is interesting and is of ecological significance. Considering the uphill task of methanol oxidation under anaerobic conditions, the studies on the present enrichment signify metabolic partnerships in the methylotrophic biochemical mechanisms operative toward energy recovery.
Similar content being viewed by others
References
Slater, J. H. (1981), inMixed Culture Fermentation, Bushell, M. E. and Slater, J. H., eds., Academic Press, New York, p. 1.
Wilkinson, T. G., Topiwala, H. H., and Hamer, G. (1974),Biotechnol. Bioeng. 16, 41.
Jones, R. D. and Hood, M. A. (1980),Microbial Ecology 6, 271.
Senior, E., Bull, A. T., and Slater, J. H. (1976),Nature 263, 476.
Kilpi, S. (1980),Microbial Ecology 6, 261.
Daughton, C. G. and Hsieh, D. P. (1977),Appl. Environ. Microbiol. 34, 175.
Gunner, H. B. and Zuckerman, B. M. (1968),Nature 217, 1183.
Hobson, P. N. (1982), inAdv. Agri. Microbiol. Subha Rao, N.S., ed., Butterworth, London, p. 523.
Lettinga G., Van Velson, A. F. M., Hobma, S. W., De Zeeuw, W., and Klapwijk, A. (1980),Biotechnol. Bioeng. 22, 699.
Hobson, P. N. Bousfield, S., and Summers, R. (1974),Crit. Rev. Environ. Control 4, 131.
Schink, B. (1989), inBiology of Anaerobic Microorganism, Zehnder, A. J. B., ed., John Wiley, New York, p. 771.
Wolin, M. J. and Miller, T. L. (1982),ASM News 48, 561.
Lalitha, K., Swaminathan, K. R., and Padma Bai, R.Appl. Biochem. Biotechnol. 47, 73.
Krishnan, S. and Lalitha, K. (1990),Appl. Biochem. Biotechnol. 26, 73.
Khan, A. W., Trottier, T. M., Patel, G. B., and Martin, S. M. (1979),J. Gen. Microbiol. 112, 365.
Weimer, A. M. and Zeikus, J. G. (1977),Appl. Environ. Microbiol. 33, 289.
Breure, A. M., Mooijman, K. A., and Van Andel, J. G. (1986),Appl. Microbiol. Biotechnol. 24, 426.
Odom, J. M. and Wall, J. D. (1983),Appl. Environ. Microbiol. 45, 1300.
Koesnander, Nishio, N., Kuroda, K., and Nagai, S. (1990),J. Ferment. Bioeng. 70, 398.
Swaminathan, K. R., Padma Bai, R., and Lalitha, K. (1993),in Adv. Pl. Biotech. Biochem, Lodha, N. L., Mehta, S. L., Ramgopal, S., and Srivastava, G. P., eds., Indian Soc. Agril. Biochemists, Kanpur, India, p. 127.
Hungate, R. E. (1969), inMethods in Microbiology, Harris, J. R. E. and Ribbons, D. W. eds., 3B, Academic, New York, p. 117.
Bryant, M. P., McBride, B. C, and Wolfe, R. S. (1968),J. Bacteriol. 95, 1118.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951),J. Biol. Chem. 193, 265.
Peck, M. W. (1989),Appl. Environ. Microbiol. 55, 940.
Haskins, E. F. and Kihara, T. (1967),Can. J. Microbiol. 13, 1283.
Albrecht, R. M., Rasmussen, D. H., Keller, C. S., and Hinsdill, R. D. (1976),J. Microscopy 108, 21.
Trüper, H. G., and Pfennig, N. (1981), inThe Prokaryotes, A Handbook on Habitats, Isolation and Identification of Bacteria. Starr, M. P., Stolp, H., Trüper, H. G., Balows, A., and Schlegel, H. G., eds., Springer-Verlag, Berlin, p. 299.
Cohen-Bazne, G., Sistrom, W. R., and Stainer, R. Y. (1957),J.Cell. Comp. Physiol. 49, 25.
Van Niel, C. B. (1944),Bacteriol. Rev. 8, 1.
Khan, A. W. (1980),FEMS Microbiol. Lett. 9, 233.
Laube, V. M. and Martin, S. M. (1981),Appl. Environ. Microbiol. 42, 413.
Pirt, S. J., Harty, D. W., Salmon, I., and Kun Lee, V. (1987),J.Ferment. Technol. 65, 159.
Siefert, E. and Pfennig, N. (1978),Appl. Environ. Microbiol. 35, 38.
Pfennig, N. (1978),Int. J. Syst. Bacteriol. 28, 283.
Jones, B. R. (1956),Sewage Ind. Wastes 28, 883.
Kobayashi, M. (1975),Prog. Water Technol. 7, 309.
Kobayashi, M. (1975), inMicrobial Energy Conversion, Schlegel, H. G. and Barnea, J., eds., E. Goltze KG, Gottingen, Germany, p. 443.
Okuda, A. and Kobayashi, M. (1963),Microbiology 32, 792.
Kimmel, D. E., Klasson, K. T., Clausen, E. C, and Gaddy, J. L. (1991),Appl. Biochem. Biotechnol. 29, 457.
Uffen, R. L. (1976),Proc. Natl. Acad. Sc. USA 73, 3298.
Hippe, H., Caspari, D., Fiebig, K., and Gottschalk, G. (1979),Proc. Natl. Acad. ACL USA 76, 494.
Jones, W. J., Nagle, D. P., Jr., and Whitman, W. D. (1987),Microbiol. Rev. 51, 135.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lalitha, K., Swaminathan, K.R., Vargheese, C.M. et al. Methanogenesis mediated by methylotrophic mixed culture. Appl Biochem Biotechnol 49, 113–134 (1994). https://doi.org/10.1007/BF02788546
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02788546