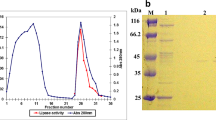
Abstract
A thermophilic soil isolate—Bacillus sp. RS-12, grew optimally at 50°C and not below 40°C. Production of an extracellular lipase by this organism was substantially enhanced when the type and concentration of carbon and nitrogen sources and initial pH of the culture medium were consecutively optimized. The lipase production was found to be growth-associated with maximum secretion in the late exponential growth phase,i.e. 15h of incubation. The enzyme activity as high as 0.98 nkat/mL was obtained under optimum conditions. Tween 80 (0.5%) and yeast extract (0.5%) were found to be the best carbon and nitrogen sources inducing maximum enzyme yield with initial pH 8.0 at 50°C. The kinetic characteristics of the crude lipase indicated the highest activity at 50–55°C and pH 8.0. It had a half life of 60, 18 and 15 min at 65, 70 and 75°C, respectively.
Similar content being viewed by others
References
Claus D., Berkeley R.C.W.: GenusBacillus Cohn 1872, pp. 1104–1139 in P.H.A. Sneath, N.S. Nair, M.E. Sharpe, J.G. Holt (Eds):Bergey's Manual of Systematic Bacteriology, Vol. 2. Williams & Wilkins, Baltimore 1986.
Gilbert E.J., Drozd J.W., Jones C.W.: Physiological regulation and optimization of lipase activity inPseudomonas aeruginosa EF2.J. Gen. Microbiol. 137, 2215–2221 (1991).
Janssen P.H., Monk C.R., Morgan H.W.: A thermophilic lipolyticBacillus sp., and continuous assay of its p-nitrophenyl palmitate esterase activity.FEMS Microbiol. Lett. 120, 195–200 (1994).
Lee S.Y., Rhee J.S.: Production and partial purification of a lipase fromPseudomonas putida 3SK.Enzyme Microb. Technol. 15, 617–623 (1993).
Lesuisse E., Schanck K., Colson C.: Purification and preliminary characterization of the extracellular lipase ofBacillus subtilis 168, an extremely basic pH-tolerant enzyme.Eur. J. Biochem. 216, 155–160 (1993).
Pabai F., Kermasha S., Morin A.: Lipase fromPseudomonas fragi CRDA 323. Partial purification, characterization and interesterification of butter fat.Appl. Microbiol. Biotechnol. 43, 42–51 (1995).
Sugihara A., Tani T., Tominaga Y.: Purification and characterization of a novel thermostable lipase fromBacillus sp.J. Biochem. 109, 211–216 (1991).
Vorderwulbecke T., Kieslich K., Erdmann H.: Comparison of lipases by different assays.Enzyme. Microb. Technol. 14, 631–639 (1992).
Vulfson E.N.: Industrial applications of lipases, pp. 271–288 in P. Wooley, S.B. Peterson (Eds):Lipases—Their Structure, Biochemistry and Application, 1st ed. Cambridge University Press, Great Britain 1994.
Wang Y., Srivastava K.C., Shen G.J., Wang H.Y.: Thermostable alkaline lipase from a newly isolated thermophilicBacillus strain A30-1 (ATCC 53841).J. Ferment. Bioeng. 79, 433–438 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sidhu, P., Sharma, R., Soni, S.K. et al. Production of extracellular alkaline lipase by a new thermophilicBacillus sp. Folia Microbiol 43, 51–54 (1998). https://doi.org/10.1007/BF02815542
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02815542