Summary
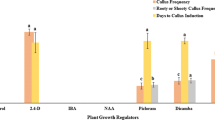
This study was conducted to establish and optimize a regeneration system for adapted U.S. rice genotypes including three commercial rice cultivars (LaGrue, Katy, and Alan) and two Arkansas breeding lines. Factors evaluated in the study were genotype, sugar type, and phytohormone concentration. The system consisted of two phases, callus induction and plant regeneration. In the callus induction phase, mature caryopses were cultured on MS medium containing either 1% sucrose combined with 3% sorbitol or 4% sucrose alone, and 0.5 to 4 mg·L−1 (2.26 to 18.10 μM) 2,4-D with or without 0.5mg·L−1) (2.32 μM) kinetin. In the plant regeneration phase, callus was transferred to 2,4-D-free MS medium containing 0 or 2 mg·L−1 (9.29 μM) kinetin combined with 0 or 0.1 mg·L−1 (0.54 μM) NAA. Callus induction commenced within a week, independent of the treatments. Callus growth and plant regeneration, however, were significantly influenced by interactions among experimental factors. Generally, the greatest callus growth and plant regeneration were obtained with 0.5 mg·L−1 (2.26 μM) 2,4-D and decreased with increasing 2,4-D concentrations. Kinetin enhanced callus growth only when combined with 0.5 mg·L−1 (2.26 μM) 2,4-D, and 4% sucrose. Inducing callus on kinetin-containing medium generally enhanced regeneration capacity in the presence of sucrose but not with a sucrose/sorbitol combination. Media containing sucrose alone generally supported more callus proliferation, but the sucrose/sorbitol combination improved regeneration of some cultivars. NAA and kinetin had little effect on regeneration.
Similar content being viewed by others
References
Abe, T.; Futsuhara, Y. Genotype variability for callus formation and plant regeneration in rice (Oryza sativa L.). Theor. Appl. Genet. 72:3–10; 1986.
Abe, T.; Futsuhara, Y. Selection of higher regenerative callus and change in isozyme pattern in rice (Oryza sativa L.). Theor. Appl. Genet. 78:648–652; 1989.
Bajaj, Y. P. S. Biotechnology in agriculture and forestry, Vol. 14, Rice. Berlin: Springer-Verlag; 1991:645 pp.
Bhaskaran, S.; Smith, R. H. Regeneration in cereal tissue culture: A review. Crop Sci. 30:1328–1336; 1990.
Bhattacharya, P.; Sen, S. K. Potentiality of leaf sheath cells for regeneration of rice (Oryza sativa L.). Theor. Appl. Genet. 58:87–90; 1980.
Brisibe, E. A.; Taniguchi, T.; Maeda, E. In vitro plant regeneration from morphogenic callus cultures of cultigens and wildOryza species. Jpn. J. Crop Sci. 59:557–565; 1990.
Brisibe, E. A.; Miyake, H.; Taniguchi, T., et al. Callus formation and scanning electron microscopy of plantlet regeneration in African rice (Oryza glaberrima Steud). Plant Sci. 83:217–224; 1992.
Chen, L.-J.; Luthe, D. S. Analysis of proteins from embryogenic and nonembryogenic rice (Oryza sativa L.) calli. Plant Sci. 48:181–188; 1987.
Guo, Y.; Liang, H.; Berns, M. W. Laser-mediated gene transfer in rice. Physiol. Plant. 93:19–24; 1995.
Heyser, J. W.; Dykes, T. A.; DeMott, K. J., et al. High frequency, long term regeneration of rice from callus culture. Plant Sci. Lett. 29:175–182; 1983.
Hiei, Y.; Ohta, S.; Komari, T., et al. Efficient transformation of rice (Oryza sativa L.) mediated byAgrobacterium and sequence analysis of boundaries of the T-DNA. Plant J. 6:217–282; 1994.
Kavi Kishor, P. B. Energy and osmotic requirement for high frequency regeneration of rice plants from long-term cultures. Plant Sci. 48:189–194; 1987.
Kothari, S. L.; Davey, M. R.; Lynch, P. T., et al. Transgenic rice. In: Kung, S.; Wu, R., ed. Transgenic plants, Vol. 2. San Diego: Academic Press, Inc.; 1993:3–20.
Mirlohi, A. F.; Thompson, L. F.; Dilday, R. H., et al. In vitro culture of several rice cultivars. Proc. Ark. Acad. Sci. 43:55–56; 1989.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15:473–497; 1962.
Oard, J. H.; Rutger, J. N. Callus induction and plant regeneration in elite U.S. rice lines. Crop Sci. 28:565–567; 1988.
Ogawa, T.; Hagio, T.; Ohkawa, Y. Plant regeneration from isolated pollen grains in Indica type rice (Oryza sativa L.). Japan. J. Breed. 42:657–679; 1992.
Ozawa, K.; Komamine, A. Establishment of a system of high-frequency embryogenesis from long-term cell suspension cultures of rice (Oryza sativa L.). Theor. Appl. Genet. 73:205–211; 1989.
Raghava, R.; Nabors, M. W. Cytokinin mediated long-term, high-frequency plant regeneration in rice tissue cultures. Z. Pflanzenphysiol. 113:315–323; 1984.
Rueb, S.; Leneman, M.; Schilperoort, R. A., et al. Efficient plant regeneration through somatic embryogenesis from callus induced on mature rice embryos (Oryza sativa L.). Plant Cell Tissue Organ Cult. 36:259–264; 1994.
Tsukahara, M.; Hirosawa, T. Characterization of factors affecting plantlet regeneration from rice (Oryza sativa L.) callus. Bot. Mag. 105:227–233; 1992a.
Tsukahara, M.; Hirosawa, T. Simple dehydration treatment promotes plantlet regeneration of rice (Oryza sativa L.) callus. Plant Cell Rep. 11:550–553; 1992b.
Wernicke, W.; Brettell, R.; Wakizuka, T., et al. Adventitious embryoid and root formation from rice leaves. Z. Pflanzenphysiol. Bd. 103:361–365; 1981.
Yoshida, K. T.; Fujii, S.; Sakata, M., et al. Control of organogenesis and embryogenesis in rice calli. Breed. Sci. 44:355–360; 1994.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Al-Khayri, J.M., Shamblin, C.E. & Anderson, E.J. Callus induction and plant regeneration of U.S. rice genotypes as affected by medium constituents. In Vitro Cell.Dev.Biol.-Plant 32, 227–232 (1996). https://doi.org/10.1007/BF02822692
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02822692