Summary
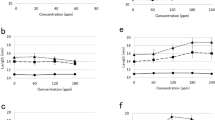
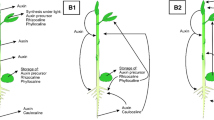
Fully habituated organogenic and nonorganogenic sugarbeet calluses reacted to application of the synthetic auxin [3-benzo(b) selenienyl] acetic acid by changes in growth and ethylene production. Treatment of fully habituated cells of periwinkle with 2,4-dichlorophenoxyacetic acid led to the decrease of free cytokinin contents (isopentenyl adenine, zeatin riboside, and zeatin) during the late exponential phase of growth. The polyamine contents were also modified and the capacity to biotransform secologanin into ajmalicine was decreased. Treatment of the habituated periwinkle cells with zeatin greatly increased the amount of a polypeptide of 16 kDa; this response was more marked than that displayed by the auxin-dependent line. These data show that hormone-independent calluses and cell suspensions can retain some sensitivity to growth hormones. However, differences of responses were observed between the auxin-dependent lines and the habituated lines.
Similar content being viewed by others
References
Binns, A. N.; Roman, G.; Teutonico, R. A. Accumulation of dehydrodiconiferyl alcohol glucoside in cytokinin habituated cell lines. In: Kaminek, M.; Mok, D. W. S.; Zazimalova, E., eds. Physiology and biochemistry of cytokinins in plants. The Hague: SPB Academic Publishing; 1992:59–64.
Bishop, J. M. The molecular genetics of cancer. Science 235:305–311; 1987.
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254; 1976.
Braun, A. C.; Morel, G. A comparison of normal, habituated and crown-gall tumor tissue implants in the European grape. Am. J. Bot. 37:499–505; 1950.
Butcher, D. N. Plant tumour cells. In: Street, H. E., ed. Plant tissue and cell culture. Oxford, England: Blackwell Scientific Publications; 19977 429–461.
Butcher, D. N. Plant tumour cells. In: Street, H. E., ed. Plant tissue and cell culture. Oxford, England: Blackwell Scientific Publications; 1977: 429–461.
Campell, B. R.; Town, C. D. Physiology of hormone autonomous tissue lines derived from radiation-induced tumours ofArabidopsis thaliana. Plant Physiol. 97:1166–1173; 1991.
Carrié, B.; Hagège, D.; Kevers, C., et al. Auxin-induced growth enhancement of a sugarbeet callus while reducing ethylene production and polyamine. Paris, France: C. R. Acad. Sci. 315:165–170; 1992.
Chen, K. H. Analysis of indole-3-acetic acid in tobacco genetic tumours and wheat GA3-insensitive mutant “Toru Thumb.” 1987. PhD Thesis, Univ. of Maryland, College Park, MD.
Christou, P. Habituation inin vitro soybean cultures. Plant Physiol. 88:809–812; 1987.
Crèvecoeur, M.; Hagège, D.; Catesson, A. M., et al. Ultrastructural characteristics of cells from normal and habituated sugar beet calli. Plant Physiol. Biochem. 30:87–95; 1992.
Décendit, A.; Liu, D.; Ouelhazi, L., et al. Cytokinin-enhanced accumulation of indole alkaloids inCatharanthus roseus cell cultures. The factors affecting the cytokinin response. Plant Cell Rep. 11:400–403; 1992.
Everett, N. P. 2,4-D-independent cell cultures of sycamore (Acer pseudoplatanus L.): isolation, responses to 2,4-D and kinetin, and sensitivities to antimetabolites. J. Exp. Bot. 32:171–182; 1981.
Gamborg, O. L.; Miller, R. A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50:151–158; 1968.
Gaspar, Th. Seleniated forms of indolylacetic acid: new powerful synthetic auxins. Acros Organics Acta. 1:65–66; 1995.
Gaspar, Th.; Hagège, D.; Kevers, et al. When plant teratomas turn into cancers in the absence of pathogens. Physiol. Plant. 83:696–701; 1991.
Gaspar, Th.; Kevers, C.; Bisbis, B., et al. Cancer végétalin vitro: aspects morphogénétiques et biochimiques. In: Dubois, J.; Demarly, Y., eds. Quel avenir pour l'amélioration des plantes. Paris, France: John Libbey Eurotext; 1995:165–171.
Gaspar, Th.; Kevers, C.; Penel, C., et al. Auxin control of calcium-mediated peroxidase secretion by auxin-dependent and auxin-independent sugarbeet cells. Phytochemistry 22:2657–2660; 1983.
Gaspar, Th.; Kevers, C.; Penel, C., et al. Biochemical characterization of normal and habituated sugarbeet calli: relationship with anatomy, habituation and organogenesis. Potsdamer Forschungen B57:20–30; 1988.
Gautheret, R. J. Hétéro-auxines et cultures de tissus végétaux. Bull. Soc. Chim. Biol. 24:13–41; 1942.
Gautheret, R. J. Sur la variabilité de propriétés physiologiques des cultures de tissus végétaux. Rev. Gén. Bot. 62:1–106; 1955.
Hagège, D. Etude comparative du cal normal et du cal habitué deBeta vulgaris L.altissima: aspects cytologiques, ultrastructuraux et biochimiques. Relations avec l'état tumoral. 1990. Thèse Doct. Univ. of Caen. Caen, France.
Hagège, D. Proto-oncogenes in plants: widespread conserved genes for which roles? Plant Physiol. Biochem. 31:621–629; 1993.
Hagège, D.; Catania, R.; Micalef, H., et al. Nuclear shape and DNA content of fully habituated nonorganogenic sugarbeet cells. Protoplasma 166:49–54; 1992a.
Hagège, D.; Kevers, C.; Boucaud, J., et al. Polyamines, phospholipids, and peroxides in normal and habituated sugarbeet calli. J. Plant Physiol. 136:641–645; 1990a.
Hagège, D.; Kevers, C.; Gaspar, Th., et al. A comparison between ethylene production, ACC and mACC contents, and hydroperoxyde level in normal and habituated sugarbeet calli. Physiol. Plant. 82:397–400; 1991.
Hagège, D.; Kevers, C.; Le Dily, et al. NaCl dependent growth rate of normal and habituated calli, ethylene production and peroxidase activity. Paris, France: C. R. Acad. Sci. 310:259–264; 1990b.
Hagège, D.; Werck-Reichart, D.; Schmitt, P., et al. Deficiency in tetrapyrrole-containing compounds in a non-organogenic habituated sugarbeet cell line. Plant Physiol. Biochem. 30:649–654; 1992b.
Hansen, C. E.; Meins, F., Jr.; Aebi, R. Hormonal regulation of zeatin riboside accumulation by cultured tobacco cells. Planta 172:520–525; 1987.
Hervagault, J. F.; Ortoleva, P. J.; Ross, J., et al. Reversal of the crown-gall tumor: an interpretation based on multiple steady state. Proc. Natl. Acad. Sci. USA 88:10797–10800; 1991.
Ikeda, M.; Ojima, M.; Oshira, K., et al. Habituation in suspension-cultured soybean cells to thiamine and its precursors. Plant Cell Physiol. 20:733–739; 1979.
Jackson, J. A.; Lyndon, R. F. Habituation: cultural curiosity or developmental determinant? Physiol. Plant. 79:579–583; 1990.
Jemmali, A.; Boxus, P.; Kevers, C., et al. Carry over of morphological and biochemical characteristics associated to hyperflowering of micropropagated strawberries. J. Plant Physiol. 147:435–440; 1995.
Kevers, C. Control by 2,4-D of auxin protectors in sugar-beet callus. Arch. Intern. Physiol. 20:733–739; 1981.
Kevers, C.; Coumans, M.; De Greef, W., et al. Habituation in sugarbeet callus: auxin content, auxin protectors, peroxidase pattern and inhibitors. Physiol. Plant. 51:281–286; 1981.
Kevers, C.; Greppin, H.; Penel, C., et al. Short- and long-term effects of auxins on ethylene production by auxin-dependent and auxin-independent sugarbeet cell lines. Arch. Intern. Physiol. Biochem. 93: B39-B40; 1985.
Kevers, C.; Sticher, L.; Penel, C., et al. Calcium-controlled peroxidase secretion by sugarbeet cell suspensions in relation to habituation. Plant Growth Regul. 1:61–66; 1982.
Kulescha, Z. Recherches sur l'élaboration de substances de croissance par les tissus végétaux. Rev. Gén. Bot. 59:19–41; 1952.
Kulescha, Z.; Gautheret, R. Sur l'élaboration de substances de croissance par 3 types de cultures de tissus de Scorzonère: cultures normales, cultures de crowngall et cultures accountumées à l'hétéro-auxine. Paris, France: C. R. Acad. Sci. 227:292–294; 1948.
Kutacek, M.; Eeder, J.; Opatrny, Z., et al. Comparison of anthranilate synthase activity and IAA content in normal and auxin-habituatedDioscorea deltoida tissue cultures. Biochem. Physiol. Pflanz. 176:244–250; 1981.
Le Dily, F.; Billard, J. P.; Gaspar, Th., et al. Disturbed nitrogen metabolism associated with the hyperhydric status of sugarbeet. Physiol. Plant. 88:129–134; 1993.
Mayer, J. E.; Hahne, G.; Palme, K., et al. A simple and general plant tissue extraction procedure for two-dimensional gel electrophoresis. Plant Cell Rep. 6:77–81; 1987.
Meins, F., Jr. Habituation of cultured plant cells. In: Kahl, G.; Schell, J. S., eds. Molecular biology of plant tumors. London: Academic Press; 1982:3–31.
Meins, F., Jr. Habituation: heritable variation in the requirement of cultured plant cells for hormones. Annu. Rev. Genet. 23:395–408; 1989.
Mérillon, J. M.; Dupéron, P.; Montagu, M., et al. Modulation by cytokinin of membrane lipids inCatharanthus roseus cells. Plant Physiol. Biochem. 1:749–755; 1993.
Mérillon, J. M.; Filali, M.; Dupéron, P., et al. Effect of 2,4-dichlorophenoxyacetic acid and habituation on lipid and protein composition of microsomal membranes from periwinkle cell suspensions. Plant Physiol. Biochem. 33:443–451; 1995a.
Mérillon, J. M.; Huguet, F.; Fauconneau, B., et al. Specific binding of verapamil to microsomal membranes ofCatharanthus roseus cell suspensions. J. Plant Physiol. 146:279–282; 1995b.
Mérillon, J. M.; Ouelhazi, L.; Doireau, P., et al. Metabolic changes and alkaloid production in habituated and non-habituated cells ofCatharanthur roseus grown in hormone-free medium: comparing hormone-deprived non-habituated cells with habituated cells. J. Plant Physiol. 134:54–60; 1989.
Mérillon, J. M.; Ramawat, K.; Andreu, F., et al. Accumulation alcaloïdique dans des lignées cellulaires deCatharanthus roseus entretenues en présence ou en l'absence de phytohormones. Paris, France: C. R. Acad. Sci. 303:689–692; 1986.
Mousdale, D. M. A.; Fidgeon, C.; Wilson, G., et al. Auxin content and growth patterns in auxin-dependent and auxin-autotrophic plant cell and tissue cultures. Biol. Plant. 27:257–264; 1985.
Nakajima, H.; Yokota, T.; Matsumoto, T., et al. Relationship between hormone content and autonomy in various autonomous tobacco cells cultured in suspension. Plant Cell Physiol. 29:1489–1499; 1979.
Nanney, D. L. Epigenetic control systems. Proc. Natl. Acad. Sci. USA 44:712–717; 1958.
Oakley, B. R.; Kirsch, D. R.; Norris, N. R., et al. A simplified ultra-sensitive silver stain for detecting proteins in polyacrylamide gel. Anal. Biochem. 105:361–363; 1980.
Ouelhazi, L.; Filali, M.; Décendit, A., et al. Differential protein accumulation in zeatin-and 2,4-D-treated cells ofCatharanthus roseus. Correlation with indole alkaloid biosynthesis. Plant Physiol. Biochem. 31:421–431; 1993.
Pengelly, W. L.; Meins, F., Jr. The relationship of indole-3-acetic content and growth of crown-gall tumour tissues of tobacco in culture. Differentiation 21:27–31; 1982.
Persinger, S. M.; Town, C. D. Isolation and characterization of hormone-autonomous tumours ofArabidopsis thaliana. J. Exp. Bot. 42:1363–1370; 1991.
Ramagli, L.; Rodriguez, L. V. Quantitation of microgram amounts of proteins in two-dimensional polyacrylamide gel electrophoresis sample buffer. Electrophoresis 6:559–563; 1985.
Ramawat, K. G.; Viel, C.; Chénieux, J. C., et al. Quaternary alkaloid production inRuta graveolens: suspension culture and habituation of some strains. Indian J. Exp. Biol. 25:471–475; 1987.
Saunders, J. W.; Daub, M. E. Shoot regeneration from hormone-autonomous callus from shoot cultures of several sugarbeet (Beta vulgaris L.) genotypes. Plant Sci. Lett. 34:219–233; 1984.
Smith, H. H. Plant genetic tumours. Prog. Exp. Tumor Res. 15:138–164; 1972.
Street, H. E. Growth in organized and unorganized systems: knowledge gained by culture of organs and tissue explants. In: Steward, F. C., ed. Plant physiology. Vol. 5B. New York: Academic Press; 1969:223–224.
Syono, K.; Fujita, T., Habituation as a tumorous state that is interchangeable with a normal state in plant cells. Int. Rev. Cytol. 152:265–299; 1994.
Syono, K.; Furuya, T. Introduction of auxin-nonrequiring tobacco calluses and its reversal by treatments with auxins. Plant Cell Physiol. 15:7–17; 1974.
Szabò, M.; Köves, E.; Somogyi, I., et al. Development of auxin autotrophy inNicotiana tabacum callus cultures. Physiol. Plant. 90:348–352; 1994.
Vankova, R.; Gaudinova, A.; Kamineck, M., et al. The effect of interaction of synthetic cytokinin and auxin on production of natural cytokinins by immobilized tobacco cells. In: Kaminek, M.; Mok, D. W. S.; Zazimalova, E., eds. Physiology and biochemistry of cytokinins in plants. The Hague: SPB Academic Publishing; 1992:395–400.
Walters, H. J. P.; Geuns, J. M. C. High speed HPLC analysis of polyamines in plant tissues. Plant Physiol. 83:232–234; 1987.
White, P. R. Neoplastic growth in plants. Q. Rev. Biol. 26:1–16; 1951.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kevers, C., Filali, M., Petit-Paly, G. et al. Habituation of plant cells does not mean insensitivity to plant growth regulators. In Vitro Cell Dev Biol - Plant 32, 204–209 (1996). https://doi.org/10.1007/BF02822767
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02822767