Abstract
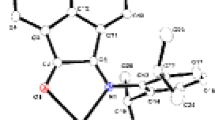
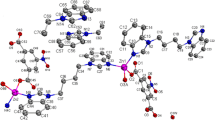
A tetranuclear zinc benzoate Zn4O(C6H5CO2)6 was synthesized and characterized by X-ray single crystal determination. It crystallizes in cubic, space groupIa-3d. Its crystal cell is very large,a=4. 100 63 (18) nm, V=68.953(5) nm3 and Z=48. The structure is composed of discrete Zn4O(C6H5CO2)6 molecules. In each molecule, four zinc atoms are held together by a central oxygen atom, which results in the formation of a regular tetrahedron. All benzoate ligands coordinate to zinc atoms in a bidentate bridging mode. Each zinc atom is in a slightly distorted tetrahedral geometry, coordinated by three benzoate oxygen atoms and the central oxygen atom. The intermolecular interactions result in the formation of a three-dimensional supramolecular framework, with non-intersecting parallel channels.
Similar content being viewed by others
References
Koyama H, Saito Y. The Crystal Structure of Zinc Oxyacetate, Zn4O(CH3COO)6.Bull Chem Soc Jpn, 1954,27(2): 112.
Stucky G, Rundle R E. The Crystal and Molecular Structure of Mg4Br6·4C4H10O, a Grignard Reagent Oxidation Product.J Am Chem Soc, 1964,86: 4821.
Bertrand J A. Five-Coordinate Complexes. III: Structure and Properties ofμ 4-Oxo-Hexa-μ-Chloro-Tetrakis {(Triphenyl-Phosphineoxide) Copper(II)},Inorg Chem, 1967,6: 495.
Castro R, Garcia-Vázquez J A, Ramero J,et al. Synthesis and Crystal Structure ofμ 4-Oxo-Hexa (μ 2-(N,S)-1-Methyl-imidazoline-2-Thionato)-Tetrazinc (II).Inorg Chim Acta, 1995,237: 143.
Tao J, Tong M L, Shi J X,et al. Blue Photoluminescent Zinc Coordination Polymers with Supertetranuclear Cores.Chem Commun, 2000, 2043.
Lee C F, Chin K F, Peng S M,et al. A luminescent Tetrameric Zinc (II) Complex Containing the 7-Azaindolate Ligand. Photophysical Properties and Crystal Structure.J Chem Soc, Dalton Trans, 1993,467.
Cotton F A, Daniels L M, Falvello L R,et al. Transition Metal (Mn, Co) and Zinc Formamidinate Compounds Having the Basic Beryllium Acetate Structure, and Unique Isomeric Iron Compounds.Inorg Chim Acta, 1997,266: 91.
Hiltunen L, Leskeiä M, Mäkelä M,et al. Orystal Structure ofμ 4-Oxo-Hexakis (μ-acetato) tetrazinc and Thermal Studies of its Precursor, Zinc Acetate Dihydrate.Acta Chem Scand Ser A, 1987,41: 548.
Kunkely H, Volger A. Absorption and Emission Spectrum of [Zn4O(Acetate)6].J C S Chem Commun, 1990, 1204.
Gordon R M, Silver H B. Preparation and Structure of Tetrazincμ 4-Oxo-Hexa-μ-Carboxylates.Can J Chem, 1983,61: 1218.
Clegg W D, Harbron R, Homan C D,et al. Crystal Structures of Three Basic Zinc Carboxylates together with Infrared and FAB Mass Spectrometry Studies in Solutions.Inorg Chim Acta, 1991,186: 51.
Sun J T, Zhang K L, Yuan L J,et al. Synthesis and Thermal Decomposition of Zinc Phthalate.Thermochim Acta, 2000,343: 105.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Supported by the National Natural Science Foundation of China (20471044)
Biography: YIN Ming-cai (1973-), female, Ph. D, research direction: functional coordination compounds.
Rights and permissions
About this article
Cite this article
Ming-cai, Y., Chi-wei, W., Chang-chun, A. et al. Synthesis and crystal structure of tetranuclear zinc benzoate. Wuhan Univ. J. Nat. Sci. 9, 939–942 (2004). https://doi.org/10.1007/BF02850804
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02850804