Abstract
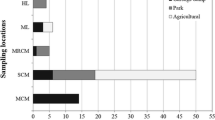
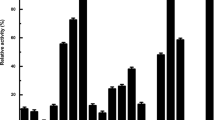
About 3,000 bacterial colonies with esterase activities were isolated from soil samples by enrichment culture and halo-size on Luria broth-tributyrin (LT) plates. The colonies were assayed for esterase activity in microtiter plates using enantiomerically pure (R)- and (S)-2-phenylbutyric acid resorufin ester (2PB-O-res) as substrates. Two enantioselective strains (JH2 and JH13) were selected by the ratio of initial rate of hydrolysis of enantiomerically pure (R)- and (S)-2-PB-O-res. When cell pellets were used, both strains showed hgh apparent enantioselectivity (E app>100) for (R)-2PB-O-res and were identified asExiguobacterium acetylicum. The JH13 strain showed high esterase activity onp-nitrophenyl acetate (pNPA), but showed low lipase activity onp-nitrophenyl palmitate (pNPP). The esterase was located in the soluble fraction of the cell extract. The crude intracellular enzyme preparation was stable at a pH range from 6.0 to 11.0.
Similar content being viewed by others
References
Sheldon, R. A. (1996) Chirotechnology: Designing economic chiral syntheses.J. Chem. Technol. Biotechnol. 67: 1–14.
Margolin, A. L. (1993) Enzymes in the synthesis of chiral drugs.Enzyme Microbiol. Technol. 15: 266–280.
Patel, R. N. (2000) Microbial/enzymatic synthesis of chiral drug intermediates.Adv. Appl. Microbiol. 47: 33–78.
Faber, K. (1996)Biotransformation on Organic Chemistry. 3rd ed., pp. 88–92. Springer, Berlin, Germany.
Topakas, E., H. Stamatis, P. Biely, D. Kekos, B. J. Macris, and P. Christakopoulos (2003) Purification and characterization of a feruloyl esterase from Fusarium oxysporum catalyzing esterification of phenolic acids in ternary water-organic solvent mixtures.J. Biotechnol. 102: 33–44.
Kovac, A., P. Stadler, L. Haalck, F. Spener, and F. Paliauf (1996) Hydrolysis and esterification of acylglycerols and analogs in aqueous medium catalyzed by microbial lipases.Biochim. Biophys. Acta 1301: 57–66.
Gokul, B., J. H. Lee, K. B. Song, T. Panda, S. K. Rhee, and C. H. Kim (2000) Screening of microorganisms producing esterase for the production of (R)-β-acetylmercaptoisobutyric acid from methyl (R,S)-β-acetylmercaptoisobutyrate.Biotechnol. Bioprocess Eng. 5: 57–60.
Sehgal, A. C. and R. M. Kelly (2003) Strategic selection of hyperthermophilic esterases for resolution of 2-arylpropionic esters.Biotechnol. Prog. 19: 1410–1416.
Kim, J. Y., G. S. Choi, I. S. Jung, Y. W. Ryu, and G. J. Kim (2003) A systematic approach for yielding a potential pool of enzymes: Practical case for chiral resolution of (R,S)-ketoprofen ethyl ester.Protein Eng. 16: 357–364.
Lee, S.-Y., B.-H. Min, S.-W. Song, S.-Y. Oh, S.-M. Lim, S.-L. Kim, and D.-I. Kim (2001) Polyacrylamide gel immobilization of porcine liver esterase for the enantioselective production of levofloxacin.Biotechnol. Bioprocess. Eng. 6: 179–182.
Coghe, S., K. Benoot, F. Delvaux, B. Vanderhaegen, and F. R. Delvaux (2004) Ferulic acid release and 4-vinylguaiacol formation during brewing and fermentation: Indications for feruloyl esterase activity inSaccharomyces cerevisiae.J. Agric. Food Chem. 52: 602–608.
Iwasaki, Y. and T. Yamane (2004) Enzymatic synthesis of structured lipids.Adv. Biochem. Eng. Biotechnol. 90: 151–171.
Chung, Y. M., C. B. Park, and S. B. Lee (2000) Partial purification and characterization of thermostable esterase from the hyperthermophilic archaeonSulfolobus solfataricus.Biotechnol. Bioprocess Eng. 5: 53–56.
Kim, C. H., J. H. Lee, J. H. Heo, O. S. Kwon, H. A. Kang, and S. K. Rhee (2004) Cloning and expression of a novel esterase gene cpoA fromBurkholderia cepacia.J. Appl. Microbiol. 96: 1306–1316.
Lambrechts, C. and P. J. Galzy (1995) Esterase activities ofBrevibacterium sp. R312 andBrevibacterium linens 62.Biosci. Biotechnol. Biochem. 59: 1464–1471.
Simões, D. C. M., D. McNeill, B. Kristiansen, and M. Mattey (1995) Extracelular esterase activity fromBacillus stearothermophilius.Biotechnol. Lett. 17: 953–958.
Landoni, M. F. and A. Soraci (2001) Pharmacology of chiral compounds: 2-Arylpropionic acid derivatives.Curr. Drug Metab. 2: 37–51.
Stephan, E., R. Rocher, J. Aubouet, G. Pourcelot, and P. Cresson (1994) Preparation of chiral indanones and dihydrocoumarins: Application to synthesis of (+)-3-(2,6-dimethoxy-phenyl) pentanoic acid.Tetrahedron Asymmetry 5: 41–44.
Mizuno, M. and T. Shioiri (1998) Reaction of carboxylic acids with diethyl phosphoro-cyanidate: A novel synthesis of homologated α-hydroxycarboxylic acids from carboxylic acids.Tetrahedron Lett. 39: 9209–9210.
Janes, L. E. and R. J. Kazlauskas (1997) A fast spectrophotometric method to measure the enantioselectivity of hydrolases.J. Org. Chem. 62: 4560–4561.
Henke, E. and U. T. Bornscheuer (1999) Directed evolution of an esterase from Pseudomonas fluorescens. Random mutagenesis by error-prone PCR or a mutator strain and identifi-cation of mutants showing enhanced enantioselectivity by a resorufin based fluore-scene assay.Bio. Chem. 380: 1029–1033.
Chen, C. S., Y. Fujimoto, G. Girdaukas, and C. J. Sih (1982) Quantitative analyses of bio-chemical kinetic resolutions of enantiomers.J. Am. Chem. Soc. 104: 7294–7299.
Koskinen, A. M. P. and A. M. Klibanov (1996)Enzymatic Reactions in Organic Media. 1st ed., pp. 172–174. Blackie Academic & Professional, London, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, BY., Kim, JH., Kim, J. et al. Screening ofExiguobacterium acetylicum from soil samples showing enantioselective and alkalotolerant esterase activity. Biotechnol. Bioprocess Eng. 10, 367–371 (2005). https://doi.org/10.1007/BF02931857
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02931857