Abstract
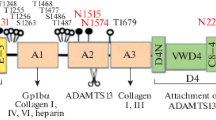
Vascular endothelial cell (EC)-produced plasma von Willebrand factor (vWF) plays a critical role in primary hemostasis through its action of anchoring platelets onto the injured denuded subendothelial matrices under high shear stress. Unusually large vWF multimers (UL-vWFMs), present in plasma immediately after release from ECs, are most biologically active, but they are soon cleaved and degraded into smaller vWFMs by a specific plasma protease, termed vWF-cleaving protease (vWF-CPase), in normal circulation. Recent studies on the relationship between UL-vWFMs and vWF-CPase, together with its autoantibody (inhibitor) have brought about a clear discrimination between thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Furthermore, a congenital deficiency of this enzyme activity has been shown to cause Upshaw-Schulman syndrome, a complex constitutional bleeding diathesis. Successful purification of vWF-CPase revealed that this enzyme is composed of a single polypeptide with a molecular mass of approximately 190 kd, and its complementary DNA cloning unambiguously indicated that it is uniquely produced in the liver and its gene is located on chromosome 9q34. The messenger RNA of vWF-CPase had a span of 4.6 kb, and its enzyme was designated ADAMTS 13. The predicted complete amino acid sequence of this enzyme consisted of 1427 residues, including a signal peptide, a short propeptide terminating in the sequence RQRR, a reprolysin-like metalloprotease domain, a disintegrin-like domain, a thrombospondin-1 repeat (TSP1), a cysteine-rich domain, an ADAMTS spacer, 7 additional TSP1 repeats, and 2 CUB domains.
Similar content being viewed by others
References
Bloom AL, Giddings JC, Wilke CJ. Factor VIII on the vascular intima: possible importance in hemostasis and thrombosis.Nature. 1973;241:217–219.
Jaffe EA, Hoyer LW, Nachman RL. Synthesis of antihemophilic factor antigen by cultured human endothelial cells.J Clin Invest. 1973;60:914–921.
Sporn LA, Chavin SI, Marder VJ, Wagner DD. Biosynthesis of von Willebrand protein by human megakaryocytes.J Clin Invest. 1985;76:1102–1106.
Matsui T, Fujimura Y, Nishida S, Titani K. Human plasma α2- macroglobulin and von Willebrand factor possess covalently linked ABO(H) blood antigens in subjects with corresponding ABO phe- notype.Blood. 1993;82:663–668.
Matsui T, Shimoyama T, Matsumoto M, et al. ABO blood group antigen on human plasma von Willebrand factor after ABO- mismatched bone marrow transplantation.Blood. 1999;94:2895–2900.
Fujimura Y, Titani K. Structure and function of von Willebrand factor. In: Bloom AL, Forbes CD, Thomas DP, Tuddenham EGD, eds.Haemostasis and Thrombosis. New York, NY: Churchill Livingstone; 1994:379–395.
Ruggeri ZM. von Willebrand factor.J Clin Invest. 1997;99:559–564.
Sadler JE. Biochemistry and genetics of von Willebrand factor.Annu Rev Biochem. 1998;67:395–424.
Furlan M. von Willebrand factor: molecular size and functional activity.Ann Hematol. 1996;72:341–348.
Furlan M, Robles R, Lämmle B. Partial purification and characterization of a protease from human plasma cleaving Von Willebrand factor to fragments produced by in vivo proteolysis.Blood. 1996;87:4223–4234.
Tsai H-M. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion.Blood. 1996;87:4235–4244.
Moschcowitz E. Hyaline thrombosis of the terminal arterioles and capillaries; a hitherto undescribed disease.Proc N Y Pathol Soc. 1924;24:21–24.
Gasser C, Gautier E, Steck A, Siebenmann RE, Oechslin R. Hämolytisch-urämische syndrome: bilaterale Nierenrinden- nekrosen bei akuten erworbenen hämolytischen Anämien.Schweiz Med Wochenschr. 1955;85:905–909.
Furlan M, Robles R, Solenthaler M, Wassmer M, Sandoz P, Lämmle B. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura.Blood. 1997;89:3097–3103.
Furlan M, Robles R, Galbusera M, et al. von Willebrand factor- cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome.N Engl J Med. 1998;339:1578–1584.
Tsai H-M, Lian EC-Y. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura.N Engl J Med. 1998;339:1585–1594.
Furlan M, Robles R, Solenthaler M, Lämmle B. Acquired deficiency of von Willebrand factor cleaving protease in a patient with thrombotic thrombocytopenic purpura.Blood. 1998;91:2839–2846.
Kinoshita S, Yoshioka A, Park Y-D, et al. Upshaw-Schulman syndrome revisited: a concept of congenital thrombotic thrombocy- topenic purpura.Int J Hematol. 2001;74:101–108.
Hershgold EJ, Sprawls S. Molecular properties of purified human, bovine and porcine antihemophilic globulin (AHG).Fed Proc. 1966;25:317.
Johnson AJ, Newman J, Howell MB, Puszkin S. Purification of anti- hemophilic factor (AHF) for clinical and experimental use.Thromb Diath Haemorrh Suppl. 1967;26:377–381.
Ratnoff OD, Kass L, Lang PD. Studies of the purification of anti- hemophilic factor (factor VIII), II: separation of partially purified antihemophilic factor by gel filtration of plasma.J Clin Invest. 1969;48:957–962.
van Mourik JA, Mochtar IA. Purification of antihemophilic factor (factor VIII) by gel chromatography.Biochim Biophys Acta. 1970;221:677–679.
Zimmerman TS, Ratnoff OD, Powell AE. Immunologic differentiation of classic hemophilia (factor VIII deficiency) and von Willebrand’s disease. With observations on combined deficiencies of antihemophilic factor and proaccelerin (factor V) and on an acquired circulating anticoagulant against antihemophilic factor.J Clin Invest. 1971;50:244–254.
Marchesi SL, Shulman NR, Gralnick HR. Studies on the purification and characterization of human factor VIII.J Clin Invest. 1972;51:2151–2161.
Legaz ME, Schmer G, Counts RB, Davie EW. Isolation and characterization of human factor VIII (antihemophilic factor).J Biol Chem. 1973;248:3946–3955.
van Mourik JA, Bouma BN, LaBruyère WT, De Graaf S, Mochtar IA. Factor VIII, a series of homologous oligomers and a complex of two proteins.Thromb Res. 1974;4:155–164.
Owen WG, Wagner RH. Antihemophilic factor: separation of an active fragment following dissociation by salts or detergents.Thromb Diath Haemorrh. 1972;27/3:502–515.
Bouma BN, Wiegerink Y, Sixma JJ, van Mourik JA, Mochtar IA. Immunologic characterization of purified antihaemophilic factor A (factor VIII) which corrects abnormal platelet retention in von Willebrand’s disease.Nature. 1972;236:104–106.
Weiss HJ, Hoyer LW. von Willebrand factor: dissociation from anti- hemophilic factor procoagulant activity.Science. 1973;182:1149–1151.
Cooper HA, Griggs TR, Wagner RH. Factor VIII recombination after dissociation by CaCl2.Proc Natl Acad Sci U S A. 1973;70:2326–2329.
Rick ME, Hoyer LW. Immunologic studies of antihemophilic factor (AHF, Factor VIII). V. Immunologic properties of AHF sub- units produced by salt dissociation.Blood. 1973;42:737–747.
Meyer D, Jenkins CSO, Dreyfus MD, Fressinaud E, Larrieu M-J. von Willebrand factor and ristocetin, II: relationship between von Willebrand factor, von Willebrand antigen and factor VIII activity.Br J Haematol. 1974;28:579–599.
Hougie C, Seargeant RB, Brown JE, Baugh RF. Evidence that factor VIII and the ristocetin aggregating factor (VIII Rist) are separate molecular entities.Proc Soc Exp Biol Med. 1974;147:58–61.
McKee PA, Andersen JC, Switzer ME. Molecular structural studies of human factor VIII.Ann N Y Acad Sci. 1974;240:8–33.
Toole JJ, Knopf JL, Wozney JM, et al. Molecular cloning of cDNA encoding human antihemophilic factor.Nature. 1984;312:342–347.
Vehar GA, Keyt B, Eaton D, et al. Structure of human factor VIII.Nature. 1984;312:337–342.
Verweij CL, de Vries CJM, Distel B, et al. Construction of cDNA coding for human von Willebrand factor using antibody probes for colony-screening and mapping of the chromosomal gene.Nucleic Acids Res. 1985;13:4699–4717.
Sadler JE, Shelton-Inlose BB, Sorace JM, Harlan JM, Titani K, Davie EW. Cloning and characterization of two cDNAs coding for human von Willebrand factor.Proc Natl Acad Sci U S A. 1985;82:6394–6398.
Ginsburg D, Handin RI, Bonthron DT, et al. Human von Wille- brand factor (vWF): isolation of complementary DNA (cDNA) clones and chromosome localization.Science. 1985;228:1401–1406.
Shelton-Inlose BB, Titani K, Sadler JE. cDNA sequences for human von Willebrand factor reveal five types of repeated domains and five possible protein sequence polymorphisms.Biochemistry. 1986;25:3164–3171.
Kaufman RJ. Biological regulation of factor VIII activity.Annu Rev Med. 1992;43:325–339.
Ruggeri ZM, Zimmerman TS. Variant von Willebrand’s disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets.J Clin Invest. 1980;65, 1318–1325.
Ruggeri ZM, Zimmerman TS. The complex multimeric composition of factor VIII/von Willebrand factor.Blood. 1981;57:1140–1143.
Chopek MW, Girma J-P, Fujikawa K, Davie EW, Titani K. Human von Willebrand factor: a multivalent protein composed of identical subunits.Biochemistry. 1986;25:3146–3155.
Girma J-P, Chopek MW, Titani K, Davie EW. Limited proteolysis of human von Willebrand factor byStaphylococcus aureus V-8: isolation and partial characterization of a platelet-binding domain.Biochemistry. 1986;25:3156–3163.
Titani K, Kumar S, Takio K, et al. Amino acid sequence of human von Willebrand factor.Biochemistry. 1986;25:3171–3184.
Wagner DD, Lawrence SO, Ohlsson-Wilheim BM, Fay PJ, Marder VJ. Topology and order of formation of interchain disulfide bonds in von Willebrand factor.Blood. 1987;69:27–32.
Ruggeri ZM, Pareti FI, Mannucci PM, Ciavarella N, Zimmerman TS. Heightened interaction between platelets and factor VIII/ von Willebrand factor in a new subtype of von Willebrand disease.N Engl J Med. 1980;302:1047–1051.
Dent JA, Berkowitz SD, Ware J, Kasper CK, Ruggeri ZM. Identification of a cleavage site directing the immunochemical detection of molecular abnormality in type IIA von Willebrand factor.Proc Natl Acad Sci U S A. 1990;87:6306–6310.
Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellums JD. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothe- lial cells in shear stress-induced platelet aggregation.J Clin Invest. 1986;78:1456–1461.
Moake JL, Turner NA, Stathopoulos NA, Nolasco L, Hellums JD. Shear-dependent platelet aggregation can be mediated by vWF released from platelets, as well as by exogenous large or unusually large vWF multimers, requires adenosine diphosphate, and is resistant to aspirin.Blood. 1988;71:1366–1374.
Ikeda Y, Handa M, Kawano K, et al. The role of von Willebrand factor and fibrinogen in platelet aggregation under varying shear stress.J Clin Invest. 1991;87:1234–1240.
Alevriadou BR, Moake JL, Turner NA, et al. Real-time analysis of shear-dependent thrombus formation and its blockade by inhibitors of von Willebrand factor binding to platelets.Blood. 1993;81:1263–1276.
Siedlecki CA, Lestini BJ, Kottke-Marchant K, Eppell SJ, Wilson DL. Shear-dependent changes in the 3-dimensional structure of human von Willebrand factor.Blood. 1996;88:2939–2950.
Tsai H-M, Sussman II, Nagel RL. Shear stress enhances the prote- olysis of von Willebrand factor in normal plasma.Blood. 1994;83:2171–2179.
Gerritsen HE, Turecek PL, Schwarz HP, Lämmle B, Furlan M. Assay of von Willebrand factor (vWF)-cleaving protease based on decreased collagen binding affinity of degraded vWF. A tool for the diagnosis of thrombotic thrombocytopenic purpura (TTP).Thromb Haemost. 1999;82:1386–1389.
Obert B, Tout H, Veyradier A, Fressinaud E, Meyer D, Girma J-P. Estimation of the von Willebrand factor-cleaving protease in plasma using monoclonal antibodies to vWF.Thromb Haemost. 1999;82:1382–1385.
Kasper CK, Aledort LM, Counts RB, et al. A more uniform measurement of factor VIII inhibitors.Thromb Diath Haemorrh. 1975;34:869–872.
Ashida A, Nakakura H, Matsumoto M, et al. An infant of TTP with a high titer inhibitor to von Willebrand factor-cleaving protease activity [abstract].Acta Paediatr Jap. 2001;105:293.
Tsai HM, Li A, Rock G. Inhibitors of von Willebrand factor- cleaving protease in thrombotic thrombocytopenic purpura.Clin Lab. 2001;46:387–392.
Tsai H-M. High titers of inhibitor of von Willebrand factor-cleaving metalloproteinase in a fatal case of acute thrombotic thrombo-cytopenic purpura.Am J Hematol. 2001;65:251–255.
Bell WR. Thrombotic thrombocytopenic purpura/hemolytic ure- mic syndrome relapse: frequency, pathogenesis, and meaning.Semin Hematol. 1997;34:134–139.
Kakishita E. Pathophysiology and treatment of thrombotic throm- bocytopenic purpura/hemolytic uremic syndrome (TTP/HUS).Int J Hematol. 2000;71:320–327.
Cines DB, Konkle BA, Furlan M. Thrombotic thrombocytopenic purpura: a paradigm shift?Thromb Haemost. 2000;84:528–535.
Yagi H, Narita N, Matsumoto N, et al. Enhanced low shear stress induced platelet aggregation by Shiga-like toxin 1 purified from Escherichia coli O157.Am J Hematol. 2001;66:105–115.
van der Plas RM, Schiphorst ME, Huizinga EG, et al. von Wille- brand factor proteolysis is deficient in classic, but not in bone marrow transplantation-associated, thrombotic thrombocytopenic purpura.Blood. 1999;93:3798–3802.
Canciani MT, Forza I, Lattuada A, Rossi E, Mannucci PM. von Willebrand factor(VWF) cleaving protease in health and disease [abstract].Thromb Haemost Suppl. 2001. Abstract 1668.
Takahashi Y, Kawaguchi C, Hanesaka Y, Fujimura Y, Yoshioka A. Plasmavon Willebrand factor-cleaving protease is low in the new- borns.Thromb Haemost Suppl. 2001;Abs 285.
Raife TJ, Atkinson BS, Montgomery RR. Comparative von Willebrand factor cleaving proteolytic activity in human saliva, serum and plasma [abstract].Blood. 2000;96:2705.
Fujikawa K, Suzuki H, McMullen B, Chung D. Purification of human vWF-cleaving protease and its identification as a new member of the metalloproteinase family.Blood. 2001;98:1662–1666.
Wolfsberg TG, Straight PD, Gerena RL, et al. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain.Develop Biol. 1995;169:378–383.
Gerritsen H, Robles R, Lämmle B, Furlan M. Partial amino acid sequence of purified von Willebrand factor-cleaving protease.Blood. 2001;98:1654–1661.
Matsumoto M, Chisuwa H, Nakazawa Y, et al. Living-related liver transplantation rescues reduced vWF-cleaving protease activity in patients with cirrhotic biliary atresia [abstract].Blood. 2000;96:636a.
Zheng X, Chung D, Takayama TH, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor cleaving protease (ADMTS13), a metalloprotease involved in thrombotic thrombo- cytopenic purpura.J Biol Chem. 2001;276:41059–41063.
Soejima K, Mimura N, Hirashima M, et al. A novel human metal- loprotease synthesized in the liver and secreted into the blood: possibly, the von Willebrand factor-cleaving protease?J Biochem. 2001;130:475–480.
Dacie JV, Mollison PL, Richardson N, Selwyn JG, Shapiro L. Atyp- ical congenital haemolytic anaemia.Q J Med. 1953;85:79–98.
Monnens LAH, Retera RJM. Thrombotic thrombocytopenic purpura in a neonatal infant.J Pediatr. 1967;71:118–123.
Wallace DC, Lovric A, Clubb JS, Carseldine DB. Thrombotic throm- bocytopenic purpura in four siblings.Am J Med. 1975;58:724–734.
Schulman I, Pierce M, Likens A, Currimbhoy Z. Studies on throm- bopoiesis, I: a factor in normal human plasma required for platelet production; chronic thrombocytopenia due to its deficiency.Blood. 1960;14:943–957.
Upshaw JD. Congenital deficiency of a factor in normal plasma that reverses microangiopathic hemolysis and thrombocytopenia.N Engl J Med. 1978;298:1350–1352.
Rennard S, Abe S. Decreased cold-insoluble globulin in congenital thrombocytopenia (Upshaw-Schulman syndrome).N Engl J Med. 1979;300:368.
Koizumi S, Miura M, Yamagami M, Horita N, Taniguchi N, Migita S. Upshaw-Schulman syndrome and fibronectin (cold-insoluble globulin).N Engl J Med. 1981;305:1284–1285.
Goodnough LT, Saito H, Ratnoff OD. Fibronectin levels in congenital thrombocytopenia: Schulman’s syndrome.N Engl J Med. 1982;306:938–939.
Shinohara T, Miyamura S, Suzuki E, Kobayashi K. Congenital microangiopathic hemolytic anemia: report of a Japanese girl.Eur J Pediatr. 1982;138:191–193.
Moake JL, Rudy C K, Troll J H, et al. Unusually large plasma factor VIII: von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura.N Engl J Med. 1982;307:1432–1435.
Moake JL, Byrnes JJ, Troll JH, et al. Effects of fresh-frozen plasma and its cryosupernatant fraction on von Willebrand factor multi- meric forms in chronic relapsing thrombotic thrombocytopenic purpura.Blood. 1985;65:1232–1236.
Miura M, Koizumi S, Nakamura K, et al. Efficiency of several plasma components in a young boy with chronic thrombocytopenia and hemolytic anemia who responds repeatedly to normal plasma infusions.Am J Hematol. 1984;17:307–319.
Hara T, Kitano A, Kajiwara T, Kondo T, Sakai K, Hamasaki Y. Factor VIII concentrate-responsive thrombocytopenia, hemolytic anemia, and nephropathy. Evidence that factor VIII:von Willebrand factor is involved in its pathogenesis.Am J Ped Hematol Oncol. 1986;8:324–328.
Karpman D, Holmberg L, Jirgard L, Lethagen S. Increased platelet retention in familial recurrent thrombotic thrombocytopenic purpura.Kidney Int. 1996;49:190–199.
Miura M, Koizumi S, Miyazaki H. Thrombopoietin in Upshaw- Schulman syndrome.Blood. 1997;89:4663–4664.
Yagi H, Konno M, Kinoshita S, et al. Plasma of patients with Upshaw-Schulman syndrome, a congenital deficiency of von Wille- brand factor-cleaving protease, enhances the aggregation of normal platelets under high shear stress.Br J Haematol. 2001;115:991–997.
Moake JL, Turner NA, Stathopoulas NA, Nolasco L, Hellums JD. Shear-induced platelet aggregation can be mediated by vWF release from platelets, as well as by exogenous large or unusually large vWF multimers, requires adenosine diphosphate, and its resistant to aspirin.Blood. 1988;71:1366–1374.
Chow TW, Hellums JD, Moake JL, Kroll M. Shear stress-induced von Willebrand factor binding to platelet glycoprotein Ib initiates calcium influx associated with aggregation.Blood. 1992;80:113–120.
Makita K, Shimoyama T, Sakurai Y, et al. Placental ecto-ATP diphosphohydrolase: its structural feature distinct from CD39, localization and inhibition on shear-induced platelet aggregation.Int J Hematol. 1998;68:297–310.
Gachet C. ADP receptors of platelets and their inhibition.Thromb Haemost. 2001;86:222–232.
Feldman JD, Mardiney MR, Uranue ER, Cutting H. The vascular pathology of thrombotic thrombocytopenic purpura. An immuno- histochemical and ultrastructural study.Lab Invest. 1966;15:927–946.
Neame PB, Lechago J, Ling ET, Koval A. Thrombotic thrombocy- topenic purpura: report of a case with disseminated intravascular platelet aggregation.Blood. 1973;42:805–814.
Asada Y, Sumiyoshi A, Hayashi T, Suzumiya J, Kaketani K. Immunohistochemistry of vascular lesion in thrombotic thrombo- cytopenic purpura, with special reference to factor VIII related antigen.Thromb Res. 1985;38:469–479.
Bull BS, Kuhn IH. The production of schistocytes by fibrin strands (a scanning electron microscope study).Blood. 1970;35:104–111.
Konno M, Yoshioka A, Takase T, Imai T. Partial clinical improvement in Upshaw-Schulman syndrome following prostacyclin infusion.Acta Paediat Jap. 1995;37:97–100.
Xie L, Chesterman CN, Hogg PJ. Reduction of von Willebrand factor by endothelial cells.Thromb Haemost. 2000;84:506–513.
Xie L, Dai CN, Chesterman CN, Hogg PJ. Thrombospondin-1 controls thehaemostatic activity of von Willebrand factor.Thromb Haemost. 2001;Abs OC85.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Fujimura, Y., Matsumoto, M., Yagi, H. et al. von Willebrand Factor—Cleaving Protease and Upshaw-Schulman Syndrome. Int J Hematol 75, 25–34 (2002). https://doi.org/10.1007/BF02981975
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02981975