Abstract
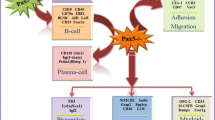
Since the first identification of interleukin (IL)-6 as a myeloma cell growth factor by Dr. Kawano’s and Dr. Klein’s groups 14 years ago, numerous studies have emphasized its major roles in the emergence of malignant plasma cells in vivo and in the generation of normal plasma cells. Four transcription factors control B-cell differentiation into plasma cells. The B-cell transcription factor pax-5 is mainly responsible for a B-cell phenotype, andbcl-6 represses the plasma cell transcription factor blimp-1 and plasma cell differentiation.bcl-6 expression is triggered by CD40 and IL-4 activation. A lack of CD40 and IL-4 activation yields a down-regulation ofbcl-6 expression, and IL-6 stimulation yields an up-regulation of blimp-1, mainly through STAT3 activation. Blimp-1 further down-regulatesbcl-6 andpax-5 expression and makes plasma cell differentiation possible. IL-6 as well as IL-10 up-regulate XBP-1. XBP-1 is another transcription factor that is involved in plasma cell differentiation and whose gene expression is shut down by pax-5.The plasma cell transcription factors blimp-1 and XBP-1 are up-regulated, and the B-cell transcription factors bcl-6 and pax-5 are down-regulated, in malignant cells compared to B-cells. Apart from the recent identification of these 4 transcription factors, the factors involved in normal plasma cell generation are mostly unknown. Regarding malignant plasma cells, 3 categories of growth factors have been identified: (1) the IL-6 family cytokines, IL-10, and interferon α that activate the Janus kinase—signal transducer and activator of transcription (JAK/STAT) and mitogen-activated protein (MAP) kinase pathways; (2) growth factors activating the phosphatidylinositol (PI)-3 kinase/AKT and MAP kinase pathways, unlike the JAK/STAT pathway (insulin-like growth factor 1, hepatocyte growth factor, and members of the epidermal growth factor family able to bind syndecan-1 proteoglycan); and (3) B-cell—activating factor (BAFF) or proliferationinducing ligand (APRIL) that activate the nuclear factor κB and PI-3 kinase/AKT pathways.BAFF and APRIL bind to BAFF receptor and TACI and are major B-cell survival factors. Recent data indicate that these various growth factors may cooperate to provide optimum signaling because they are localized together and with cytoplasmic transduction elements in caveolinlinked membrane caveolae. The identification of these myeloma cell growth factors and of the associated transduction pathways should provide novel therapeutic targets in multiple myeloma.
Similar content being viewed by others
References
Jego G, Robillard N, Puthier D, et al. Reactive plasmacytoses are expansions of plasmablasts retaining the capacity to differentiate into plasma cells.Blood. 1999;94:701–712.
Tarte K, De Vos J, Thykjaer T, et al. Generation of polyclonal plasmablasts from peripheral blood B cells: a normal counterpart of malignant plasmablasts.Blood. 2002;100:1113–1122.
Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells.Annu Rev Immunol. 2003;21:205–230.
Jung J, Choe J, Li L, Choi YS. Regulation of CD27 expression in the course of germinal center B cell differentiation: the pivotal role of IL-10.Eur J Immunol. 2000;30:2437–2443.
Agematsu K, Nagumo H, Oguchi Y, et al. Generation of plasma cells from peripheral blood memory B cells: synergistic effect of interleukin-10 and CD27/CD70 interaction.Blood. 1998;91:173–180.
Yamasaki K, Taga T, Hirata Y, et al. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor.Science. 1988;241:825–828.
Suematsu S, Matsuda T, Aozasa K, et al. IgG1 plasmacytosis in interleukin 6 transgenic mice.Proc Natl Acad Sci U S A. 1989;86:7547–7551.
Kopf M, Baumann H, Freer G, et al. Impaired immune and acutephase responses in interleukin-6-deficient mice.Nature. 1994;368:339–342.
Wen XY, Stewart AK, Sooknanan RR, et al. Identification of c-myc promoter-binding protein and X-box binding protein 1 as interleukin-6 target genes in human multiple myeloma cells.Int J Oncol. 1999;15:173–178.
Reimold AM, Iwakoshi NN, Manis J, et al. Plasma cell differentiation requires the transcription factor XBP-1.Nature. 2001;412:300–307.
Hirano T. Interleukin 6 and its receptor: ten years later.Int Rev Immunol. 1998;16:249–284.
Kawano M, Hirano T, Matsuda T, et al. Autocrine generation and essential requirement of BSF-2/IL-6 for human multiple myeloma.Nature. 1988;332:83–85.
Klein B, Zhang XG, Jourdan M, et al. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6.Blood. 1989;73:517–526.
Zhang XG, Bataille R, Widjenes J, Klein B. Interleukin-6 dependence of advanced malignant plasma cell dyscrasias.Cancer. 1992;69:1373–1376.
Klein B, Wijdenes J, Zhang XG, et al. Murine anti-interleukin-6 monoclonal antibody therapy for a patient with plasma cell leukemia.Blood. 1991;78:1198–1204.
Bataille R, Barlogie B, Lu ZY, et al. Biologic effects of antiinterleukin-6 (IL-6) murine monoclonal antibody in advanced multiple myeloma.Blood. 1995;86:685–691.
Lu ZY, Brailly H, Wijdenes J, Bataille R, Rossi JF, Klein B. Measurement of whole body interleukin-6 (IL-6) production: prediction of the efficacy of anti-IL-6 treatments.Blood. 1995;86:3123–3131.
Bataille R, Jourdan M, Zhang XG, Klein B. Serum levels of interleukin-6, a potent myeloma cell growth factor, as a reflection of disease severity in plasma cell dyscrasias.J Clin Invest. 1989;84:2008–2011.
Gaillard JP, Bataille R, Brailly H, et al. Increased and highly stable levels of functional soluble interleukin-6 receptor in sera of patients with monoclonal gammopathy.Eur J Immunol. 1993;23:820–824.
Portier M, Rajzbaum G, Zhang XG, et al. In vivo interleukin-6 gene expression in the tumoral environment in multiple myeloma.Eur J Immunol. 1991;21:1759–1762.
Costes V, Portier M, Lu ZY, Rossi JF, Bataille R, Klein B. Interleukin-1 in multiple myeloma: producer cells and their role in the control of IL-6 production.Br J Haematol. 1998;103:1152–1160.
Hinson RM, Williams JA, Shacter E. Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: possible role of cyclooxygenase-2.Proc Natl Acad Sci U S A. 1996;93:4885–4890.
Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion.Blood. 1993;82:3712–3720.
Lokhorst HM, Lamme T, de Smet M, et al. Primary tumor cells of myeloma patients induce interleukin-6 secretion in long-term bone marrow cultures.Blood. 1994;84:2269–2277.
Zhang XG, Gaillard JP, Robillard N, et al. Reproducible obtaining of human myeloma cell lines as a model for tumor stem cell study in human multiple myeloma.Blood. 1994;83:3654–3663.
Suematsu S, Matsuda T, Aozasa K, et al. IgG1 plasmacytosis in interleukin-6 transgenic mice.Proc Natl Acad Sci U S A. 1989;86:7547–7551.
Suematsu S, Matsusaka T, Matsuda T, et al. Generation of plasmacytomas with the chromosomal translocation t(12;15) in interleukin 6 transgenic mice.Proc Natl Acad Sci U S A. 1992;89:232–235.
Lattanzio G, Libert C, Aquilina M, et al. Defective development of pristane-oil-induced plasmacytomas in interleukin-6-deficient BALB/c mice.Am J Pathol. 1997;151:689–696.
Zhang XG, Gu ZJ, Lu ZY, et al. Ciliary neurotropic factor, interleukin 11, leukemia inhibitory factor, and oncostatin M are growth factors for human myeloma cell lines using the interleukin 6 signal transducer gp130.J Exp Med. 1994;179:1337–1342.
Klein B. Growth factors in the pathogenesis of multiple myeloma. In: Garthon L, Durie BGM, eds.Multiple Myeloma. London: Edward Arnold Publishers; 1996:73–82.
Jourdan M, Zhang XG, Portier M, Boiron JM, Bataille R, Klein B. IFN-alpha induced autocrine production of IL-6 in myeloma cell lines.J Immunol. 1991;147:4402–4407.
Ferlin-Bezombes M, Jourdan M, Liautard J, Brochier J, Rossi JF, Klein B. IFN-alpha is a survival factor for human myeloma cells and reduces dexamethasone-induced apoptosis.J Immunol. 1998;161:2692–2699.
Arora T, Jelinek DF. Differential myeloma cell responsiveness to interferon-alpha correlates with differential induction of p19(INK4d) and cyclin D2 expression.J Biol Chem. 1998;273:11799–11805.
Lu ZY, Zhang XG, Wijdenes J, et al. Interleukin-10 is a growth factor for human myeloma cells.Blood. 1995;85:2521–2527.
Gu ZJ, Costes V, Lu ZY, et al. Interleukin-10 is a growth factor for human myeloma cells by induction of an oncostatin M autocrine loop.Blood. 1996;88:3972–3986.
De Vos J, Jourdan M, Tarte K, Jasmin C, Klein B. JAK2 tyrosine kinase inhibitor tyrphostin AG490 downregulates the mitogenactivated protein kinase (MAPK) and signal transducer and activator of transcription (STAT) pathways and induces apoptosis in myeloma cells.Br J Haematol. 2000;109:823–828.
Jourdan M,Vos JD, Mechti N, Klein B. Regulation of Bcl-2-family proteins in myeloma cells by three myeloma survival factors: interleukin-6, interferon-alpha and insulin-like growth factor 1.Cell Death Differ. 2000;7:1244–1252.
Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells.Immunity. 1999;10:105–115.
Puthier D, Derenne S, Barille S, et al. Mcl-1 and bcl-xL are co-regulated by IL-6 in human myeloma cells.Br J Haematol. 1999;107:392–395.
Derenne S, Monia B, Dean NM, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells.Blood. 2002;100:194–199.
Jourdan M, Veyrune JL, Vos JD, Redal N, Couderc G, Klein B. A major role for Mcl-1 antiapoptotic protein in the IL-6-induced survival of human myeloma cells.Oncogene. 2003;22:2950–2959.
Tu Y, Gardner A, Lichtenstein A. The phosphatidylinositol 3-kinase/ AKT kinase pathway in multiple myeloma plasma cells: roles in cytokine-dependent survival and proliferative responses.Cancer Res. 2000;60:6763–6770.
Pfeffer LM, Mullersman JE, Pfeffer SR, Murti A, Shi W, Yang CH. STAT3 as an adapter to couple phosphatidylinositol 3-kinase to the IFNAR1 chain of the type I interferon receptor.Science. 1997;276:1418–1420.
Mitsiades CS, Mitsiades N, Poulaki V, et al. Activation of NF-kappaB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications.Oncogene. 2002;21:5673–5683.
Qiang YW, Kopantzev E, Rudikoff S. Insulinlike growth factor-I signaling in multiple myeloma: downstream elements, functional correlates, and pathway cross-talk.Blood. 2002;99:4138–4146.
Pene F, Claessens YE, Muller O, et al. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma.Oncogene. 2002;21:6587–6597.
Georgii-Hemming P, Wiklund HJ, Ljunggren O, Nilsson K. Insulinlike growth factor I is a growth and survival factor in human multiple myeloma cell lines.Blood. 1996;88:2250–2258.
Jelinek DF, Witzig TE, Arendt BK. A role for insulin-like growth factor in the regulation of IL-6-responsive human myeloma cell line growth.J Immunol. 1997;159:487–496.
Ferlin M, Noraz N, Hertogh C, Brochier J, Taylor N, Klein B. Insulin-like growth factor induces the survival and proliferation of myeloma cells through an interleukin-6-independent transduction pathway.Br J Haematol. 2000;111:626–634.
Ge NL, Rudikoff S. Insulin-like growth factor I is a dual effector of multiple myeloma cell growth.Blood. 2000;96:2856–2861.
Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma.Oncogene. 2001;20:5991–6000.
Hsu JH, Shi Y, Hu L, Fisher M, Franke TF, Lichtenstein A. Role of the AKT kinase in expansion of multiple myeloma clones: effects on cytokine-dependent proliferative and survival responses.Oncogene. 2002;21:1391–1400.
Ge NL, Rudikoff S. Expression of PTEN in PTEN-deficient multiple myeloma cells abolishes tumor growth in vivo.Oncogene. 2000;19:4091–4095.
Standal T, Borset M, Lenhoff S, et al. Serum insulinlike growth factor is not elevated in patients with multiple myeloma but is still a prognostic factor.Blood. 2002;100:3925–3929.
Duan C. Specifying the cellular responses to IGF signals: roles of IGF-binding proteins.J Endocrinol. 2002;175:41–54.
De Vos J, Couderc G, Tarte K, et al. Identifying intercellular signaling genes expressed in malignant plasma cells by using complementary DNA arrays.Blood. 2001;98:771–780.
Wijdenes J, Vooijs WC, Clement C, et al. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1.Br J Haematol. 1996;94:318–323.
Costes V, Magen V, Legouffe E, et al. The Mi15 monoclonal antibody (anti-syndecan-1) is a reliable marker for quantifying plasma cells in paraffin-embedded bone marrow biopsy specimens.Hum Pathol. 1999;30:1405–1411.
Zimmermann P, David G. The syndecans, tuners of transmembrane signaling.Faseb J. 1999;13:S91-S100.
Wang YD, De Vos J, Jourdan M, et al. Cooperation between heparin-binding EGF-like growth factor and interleukin-6 in promoting the growth of human myeloma cells.Oncogene. 2002;21:2584–2592.
Davis-Fleischer KM, Besner GE. Structure and function of heparin-binding EGF-like growth factor (HB-EGF).Front Biosci. 1998;3:d288–299.
Normanno N, Bianco C, De Luca A, Salomon DS. The role of EGFrelated peptides in tumor growth.Front Biosci. 2001;6:D685–707.
Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy.Oncogene. 2000;19:6550–6565.
Derksen PW, Keehnen RM, Evers LM, van Oers MH, Spaargaren M, Pals ST. Cell surface proteoglycan syndecan-1 mediates hepatocyte growth factor binding and promotes Met signaling in multiple myeloma.Blood. 2002;99:1405–1410.
Seidel C, Borset M,Turesson I, Abildgaard N, Sundan A, Waage A. Elevated serum concentrations of hepatocyte growth factor in patients with multiple myeloma. The Nordic Myeloma Study Group.Blood. 1998;91:806–812.
Hjertner O, Torgersen ML, Seidel C, et al. Hepatocyte growth factor (HGF) induces interleukin-11 secretion from osteoblasts: a possible role for HGF in myeloma-associated osteolytic bone disease.Blood. 1999;94:3883–3888.
Avet-Loiseau H, Li JY, Facon T, et al. High incidence of translocations t(11;14)(q13;q32) and t(4;14)(p16;q32) in patients with plasma cell malignancies.Cancer Res. 1998;58:5640–5645.
Rasmussen T, Hudlebusch HR, Knudsen LM, Johnsen HE. FGFR3 dysregulation in multiple myeloma: frequency and prognostic relevance.Br J Haematol. 2002;117:626–628.
Chesi M, Nardini E, Brents LA, et al. Frequent translocation t(4;14)(p16.3;q32.3) in multiple myeloma is associated with increased expression and activating mutations of fibroblast growth factor receptor 3.Nat Genet. 1997;16:260–264
Sato N, Hattori Y, Wenlin D, et al. Elevated level of plasma basic fibroblast growth factor in multiple myeloma correlates with increased disease activity.Jpn J Cancer Res. 2002;93:459–466.
Hart KC, Robertson SC, Donoghue DJ. Identification of tyrosine residues in constitutively activated fibroblast growth factor receptor 3 involved in mitogenesis, Stat activation, and phosphatidylinositol 3-kinase activation.Mol Biol Cell. 2001;12:931–942.
Chesi M, Brents LA, Ely SA, et al. Activated fibroblast growth factor receptor 3 is an oncogene that contributes to tumor progression in multiple myeloma.Blood. 2001;97:729–736.
Pollett JB, Trudel S, Stern D, Li ZH, Stewart AK. Overexpression of the myeloma-associated oncogene fibroblast growth factor receptor 3 confers dexamethasone resistance.Blood. 2002;100:3819–3821.
Mackay F, Kalled SL. TNF ligands and receptors in autoimmunity: an update.Curr Opin Immunol. 2002;14:783–790.
Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells.Nat Rev Immunol. 2002;2:465–475.
Claudio JO, Masih-Khan E, Tang H, et al. A molecular compendium of genes expressed in multiple myeloma.Blood. 2002;100:2175–2186.
Tarte K, Moreaux J, Legouffe E, Rossi JF, Klein B. BAFF is a survival factor for multiple myeloma cells.Blood. 2002
French JD, Walters DK, Jelinek DF. Transactivation of gp130 in myeloma cells.J Immunol. 2003;170:3717–3723.
Walters DK, French JD, Arendt BK, Jelinek DF. Atypical expression of ErbB3 in myeloma cells: cross-talk between ErbB3 and the interferon-alpha signaling complex.Oncogene. 2003;22:3598–3607.
Podar K, Tai YT, Cole CE, et al. Essential role of caveolae in interleukin-6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells.J Biol Chem. 2003;278:5794–5801.
Charrin S, Le Naour F, Oualid M, et al. The major CD9 and CD81 molecular partner. Identification and characterization of the complexes.J Biol Chem. 2001;276:14329–14337.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Klein, B., Tarte, K., Jourdan, M. et al. Survival and Proliferation Factors of Normal and Malignant Plasma Cells. Int J Hematol 78, 106–113 (2003). https://doi.org/10.1007/BF02983377
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02983377