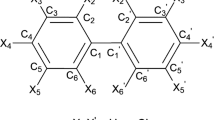
Abstract
The internal barrier of rotation (Erot) was calculated for all 209 polychlorinated biphenyls (PCBs) by using a semi-empirical method, viz. the Austin Model 1 (AMI) Hamiltonian. The difference in total energy between a forced planar state and an optimised twisted structure was defined as Erot. The Erot values were in the range of 8.33 to 483 kj/mol, and were significantly influenced by the number of chlorine atoms inortho position. An additional structural characteristic of the PCBs influencing Erot ofortho substituted congeners was substitution by chlorine atoms in vicinalmeta positions, which is assumed to prevent outward bending ofortho substituents. This so-called buttressing effect contributed with 4 to 31 kj/mol per added chlorine atom. In conclusion, the internal barrier of rotation, calculated for all 209 PCBs, provides an important structure dependent physico-chemical parameter for multivariate modelling of future quantitative structure-activity and structure-property relationships (QSARs/QSPRs).
Similar content being viewed by others
References
Mcfarland, V.A.;Clarke, J.U. Environ. Health Perspect. (1989), 81, 225–239
Safe, S. Crit. Rev. Toxicol. (1994), 24(2), 87–149
Goldstein, J.A.;Safe, S. In Halogenated Biphenyls, Terphenyls, Naphtalenes, Dibenzodioxins and Related Products;Kimbrough, R.D.;Jensen, S., Eds. Elsevier Science Publishers, The Netherlands, (1989), pp 239–286
Dynes, J.J.;Baudais, F.L.;Boyd, R.K. Can. J. Chem. (1985), 63, 1292–1299
Almenningen, A.;Bastiansen, O.;Gundersen, S.;Samdal, S.;Skancke, A. J. Mol. Struct. (1985), 128, 95–114
Bastiansen, O. Acta Chem. Scand. (1950), 4, 926–936
Romming, C.;Seip, H.M.; Aanesen Öymo, I.-M. Acta Chem. Scand. (1974), A28, 507–514
Tang, T-H.;Nowakowska, M.;Guillet, J.E.;Csizmadia, I.G. J. Mol. Struct. (1991), 232, 133–146
Zimmermann, R.;Weickhardt, C.;Boesl, U.;Schlaug, E.W. J. Mol. Struct. (1994), 327, 81–97
Sassa, S.;Sugita, O.;Ohnuma, N.;Imajo, S.;Okumura, T.;Noguchi, T.;Kappas, A. Biochem. J. (1986), 235, 291–296
Cuixen, J.M.;Kaiser, K.L.E. In QSAR in Environmental Toxicology;Kaiser, K.L.E., Eds. Kluwer Academic Publishers, Dordrecht, The Netherlands, (1984), pp 39–66
McKinney, J.D.;Gottschalk, K.E.;Pedersen, L. J. Mol. Struct. (1983), 104, 445–450
Kaiser, K.L.E. Environ. Pollut. (1974), 7, 93–101
Shaw, G.R.;Connell, D.W. Environ. Sci. Technol. (1984), 18, 18–23
Cortes, A.;Riego, J.;Paya-Perez, A.B.;Larsen, B. Toxicol. Environ. Chem. (1991), 31-32, 79–86
Falconer, R.L.;Bidleman, T.F. Atmospheric Environment, (1994), 28 (3), 547–554
Andersson, P.;Haglund, P.;Rappe, C.;Tysklind, M. J. Chemometrics. (1996), 10, 171–185
Mulholland, J.A.;Sarofim, A.F.;Rutledge, G.C. J. Phys. Chem. (1993), 97, 6890–6896
Dewar, M.J.S.;Zoebich, E.G.;Healy, E.F.;Stewart, J.J.P. J. Am. Chem. Soc. (1985), 107, 3902
HyperChem™. Release 2 for Windows, Reference Manual. Sausalito, CA, USA, (1992)
Ballschmiter, K.;Zell, M. Fresenius Z. Anal. Chem. (1980), 302, 20–31
Schulte, E.;Malisch, R. Fresenius Z. Anal. Chem. (1983), 319, 545–551
Schurig, V.;Glausch, A.;Fluck, M. Tetrahedron: Asymmetry. (1995), 6, 2161–2164
Almenningen, A.;Hartmann, A.O.;Seip, H.M. Acta Chem. Scand. (1968), 22, 1013–1024
Eliel, EX.;Wilen, S.H. Stereochemistry of Organic Compounds. Wiley, New York, USA, (1994), pp 1142–1144
Rapaport, R.A.;Eisenreich, S.J. Environ. Sci. Technol. (1984), 18, 163–170
Hawker, D.W.;Connell, D.W. Environ. Sci. Technol. (1988), 22, 382–387
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Andersson, P.L., Haglund, P. & Tysklind, M. The internal barriers of rotation for the 209 polychlorinated biphenyls. Environ. Sci. & Pollut. Res. 4, 75–81 (1997). https://doi.org/10.1007/BF02986283
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02986283