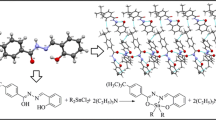
Abstract
A brief account is given of the synthesis, structural chemistry and the antibacterial, antifungal and cytotoxic effects of organotin complexes of 2-[(2,4-dichloroanilinocarbonyl)]benzoic acid. The unimolar and bimolar substitution products have been characterized by elemental analysis and spectral studies, including IR, 1H NMR, 13C NMR, 119Sn NMR, and mass spectra. The data support the binding of the oxygen atom to the tin atom in [R2Sn(OOCR’)2] and [R3Sn(OOCR’)] (R = Me, Bu, and Ph, R’ = 2-[(2,4-dichloroanilinocarbonyl)]benzoic acid). Based on these studies, with a coordination number of four, a distorted tetrahedral geometry has been proposed for the resulting derivatives in solution. The free ligand (R’/COOH) and its respective tin complexes were tested in vitro against a number of microorganisms to assess their biocidal properties and to correlate them with the structures of the derivatives.
Similar content being viewed by others
References
S.J. Blunden, P.A. Cusak, R. Hill, The Industrial Uses of Tin Chemicals, Royal Society of Chemistry, London, 1985.
K.C. Molloy, in: P.G. Harrison (Ed.), The Chemistry of Tin, Blackie Glasgow, 1989.
K.C. Joshi, V.N. Pathak, P. Panwar, Agric. Biol. Chem. 41 (1977) 543.
P.J. Smith (Ed.), Chemistry of Tin, 2nd ed. Blackie, London, ed]1998.
A.G. Davis, P.J. Smith, in: F.G.A. Stone, E.W. Abel (Eds.), Comprehensive Organometallic Chemistry G. Wilkinson, Pergamon Press, Oxford, 1982, p. 521.
M. Gielen, Appl. Organomet. Chem. 16 (2002) 481.
F. Ahmed, M. Pervez, S. Ali, M. Mazhar, A. Munir, Synth. React.Inorg.Met.-Org. Chem. 32 (2002) 665.
S. Ali, M.N. Khokhar, M.H. Bhatti, M. Mazhar, M.T. Masood, K. Shahid, A. Badshah, Synth. React. Inorg. Met.-Org. Chem. 32 (2002) 1373.
F. Ahmad, S. Ali, M. Parvez, A. Munir, M. Mazhar, K.M. Khan, T.A. Shah, Heteroatom Chem.13 (2002) 638.
S. Ali, F. Ahmad, M. Mazhar, A. Munir, M.T. Masood, Synth. React. Inorg. Met.-Org. Chem. 32 (2001) 357.
K. Shahid, S. Ali, S. Shahzadi, A. Badshah, K.M. Khan, G.M. Maharvi, Synth. React. Inorg. Met.-Org. Chem. 3 (2003) 1221.
K. Shahid, S. Ali, S. Shahzadi, Z. Akhtar, Turk. J. Chem. 27 (2003) 209.
H. Masood, S. Ali, M. Mazhar, S. Shahzadi, K. Shahid, Turk. J. Chem. 28 (2004) 75.
W.L.F. Armergo, C.L.L. Chai, Purification of Laboratory Chemicals, 5th ed; Elsevier USA, 2003.
B.N. Meyer, N.R. Ferrigni, J.E. Putnam, L.B. Jacobson, D.E. Nichols, J.L. McLaughlin, Planta Med. 45 (1982) 31.
W.S.J. Abbott, J. Econ. entomol. 18 (1925) 265.
D.J. Finney, Probit Analysis, 3rd ed. Cambridge University, 1971.
H. Blank, G. Rewbell, Arch Derm. 92 (1965) 319
S.S. Shaukat, N.A. Khan, F. Ahmed, Pak. J. Bot. 12 (1980) 97.
A. Rahman, M.I. Choudhary, W.J. Thomsen, Bioassay Techniques for Drug Development, Hardward Academic Press, Amsterdam (2001) 14.
Y. Maeda, R. Okawara, J. Organomet. Chem. 10 (1967) 247.
L.Q. Xie, Z.Q. Yang, Z. X. Zhang, D.K. Zhang, Appl. Organomet. Chem. 6 (1992) 193.
Q. Xie, Z. Yang, L. Jiang, Main Group Met. Chem.19 (1996) 509.
M. Gielen, E. Joosen, T. Mancilla, K. Jurkschat, R. Willem, C. Roobol, J. Bernheim, G. Atassi, F. Huber, E. Hoffmann, H. Prent, B. Mahieu, Main Group Met. Chem. 18 (1995) 27.
H.O. Kalinowski, S. Berger, S. Brown, 13C NMR Spectroskpie. Thieme, Stuttgartt, Germany, 1984.
S. Ahmad, S. Ali, F. Ahmad, M.H. Bhatti, A. Badshah, M. Mazhar, K.M. Khan, Synth. React. Inorg. Met.-Org. Chem. 32 (2002) 1521.
M. Jain, S. Gaur, V.P. Singh, R.V. Singh, Appl. Organomet. Chem. 18 (2004) 73.
C. Sexena, R.V. Singh, Phosphorus Sulfur Silicon. 97 (1994) 17.
M. Jain, S. Gaur, V.P. Singh, R.V. Singh, Appl. Organomet. Chem. 18 (2004) 73.
K.C. Molloy, in: F.E. Hartley (Ed.), Bioorganotin Compounds, The Chemistry of Metal- Carbon Bond, Wiley, New York, 1989.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahzadi, S., Shahid, K., Ali, S. et al. Organotin(IV) derivatives as biocides: An investigation of structure by IR, solution NMR, electron impact MS and assessment of structure correlation with biocidal activity. JICS 2, 277–288 (2005). https://doi.org/10.1007/BF03245931
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF03245931