Abstract
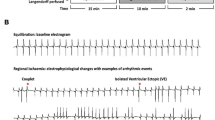
Background: The different influences of one of the PRL isoforms (PRL I) on the cardiovascular system have been described in the past. Aim: Our goal was to establish an appropriate iv dose of 2 PRL isoforms (PRL I and PRL II) in intact rats. After establishing this dose, PRL I (0.01 mg/kg) or PRL II (0.001 mg/kg) was administered in bolus 10 min before left anterior descending coronary artery occlusion (7 min) followed by re-perfusion (15 min). We then aimed to study and compare the effects of these isoforms on ischemia- and re-perfusion-induced arrhythmias in the ischemia and re-perfusion-induced arrhythmias model in rats. Materials and methods: Mortality index, ventricular fibrillation and tachycardia (VF, VT) incidence and duration, systolic, diastolic, and mean arterial blood pressure, heart rate and myocardial index of oxygen consumption [pressure rate product (PRP)] were measured and calculated. Results: Both PRL isoforms reduced animal mortality (from 50 to 18.75 and 25%, respectively). PRL II significantly reduced VF incidence (to 25%) as well as VT duration (18.21±3.09) and these effects were markedly different from PRL I and from the control group (p<0.05). Both PRL reduced PRP in the recovery phase (p<0.05). Conclusions: We proved that supra-physiological doses of PRL isoforms administered in bolus could protect against sudden cardiac death as well as severe arrhythmias episodes during re-perfusion. Because of PRL’s positive influence on the cardiovascular system and as an endogenous, well-tolerated substance, it might be of potential clinical use.
Similar content being viewed by others
References
Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function and regulation of secretion. Physiol Res 2000, 80: 1523–631.
Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu Rev Physiol 2002, 64: 47–67.
Reuwer AQ, Twickler MT, Hutten BA, et al. Prolactin levels and risk of future coronary artery disease in apparently healthy men and women. Circ Cardiovasc Genet 2009, 2: 389–95.
Kelly PA, Djiane J, Postel-Vinay MC, Edery M. The prolactin/growth hormone receptor family. Endocrine Rev 1991, 12: 235–51.
Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 1998, 19: 225–68.
Bauman DE, Eppard PJ, DeGeeter MJ, Lanza GM. Responses of high producing dairy cows to long treatment with pituitary somatotropin and recombinant somatotropin. J Dairy Sci 1985, 68: 1352–62.
Blume A, Torner L, Liu Y, Subburaju S, Aguilera G, Neumann ID. Prolactin activates mitogen-activated protein kinase signaling and corticotrophin realizing hormone transcription in rat hypothalamic neurons. Endocrinology 2009, 150: 1841–9.
Tòth BE, Homicskò K, Radnai B, et al. Salsolinol is a putative endogenous neuro-intermediate lobe prolactin-releasing factor. J Neuroendocrinol 2001, 13: 1042–50.
Yamada T, Mochiduki A, Sugimoto Y, Itoi K, Inoue K. Prolactin-releasing peptide regulates the cardiovascular system via corticotrophin-releasing hormone. J Neuroendocrinol 2009, 21: 586–93.
Os I, Kjeldsen SE, Westheim A, et al. Decreased central dopaminergic activity in essential hypertension. J Hypertens 1987, 5: 191–7.
Hudziak G, Kuska J. [Effect of bromocriptine on arterial blood pressure and serum prolactin level in patients with essential arterial hypertension]. Kardiol Pol 1988, 31: 22–9.
Horrobin DF, McNeilly AS, Jackson FS, et al. Prolactin and myocardial infarction. Lancet 1973, 302: 1261.
Horrobin DF, Manku MS, Nassar BA, Reed JD, Tynan M. Actions of prolactin and frusemide on cardiac rate and rhythm. J Physiol 1974, 239: 24P-5P.
Saito I, Takeshita E, Hayashi S, et al. Effects of captopril on plasma prolactin in patients with essential hypertension. Angiology 1990, 41: 377–81.
Haznedaroğlu IC, Tokgözoğlu L, Cağlar M, et al. The effect of chronic angiotensin converting enzyme inhibition on circulating prolactin in systemic hypertension. Horm Metab Res 1994, 26: 491–3.
Stumpe K, Higuchi M, Kolloch R, Kruck F. Hyperprolactinaemia and antihypertensive effect of bromocriptine in essential hypertension. Identification of abnormal central dopamine control. Lancet 1977, 2: 211–4.
Kuret JA, Murad F. Adenophyseal hormones and related substances. In: Gilman AG, Rall TW, Nies AS, Taylor P (ed) Goodman and Gilman’s The pharmacological basis of therapeutics. Pergamon Press, New York 1990, 1334–61.
Cooke NE, Coit D, Weiner RI, Baxter JD, Martial JA. Structure of cloned DNA complementary to rat prolactin messenger rRNA. J Biol Chem 1980, 255: 6502–10.
Selye H, Bajusz E, Grasso S, Mendell P. Simple techniques for the surgical occlusion of coronary vessels in the rat. Angiology 1960, 11: 398–407.
Clark C, Foreman MI, Kane KA, McDonald FM, Parratt JR. Coronary artery ligation in anesthetized rats as a method for the production of experimental dysrhythmias and for the determination of infarct size. J Pharmacol Meth 1980, 3: 357–68.
Crome R, Hearse DJ, Manning AS. Ischemia- and reperfusion-induced arrhythmias: beneficial actions of nifedipine. J Cardiovasc Pharmacol 1986, 8: 1249–56.
Krzemi ski TF, Grzyb J, Porc MP, Chatterjee SS. Anti-arrhythmic cardio-protective effects of furnidipine in rat model: A dose response study. Eur J Pharmacol 2006, 549: 91–7.
Walker MJ, Curtis MJ, Hearse DJ, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia, infarction, and re-perfusion. Cardiovasc Res 1988, 22: 447–55.
Ryszka F, Ludyga K, Strokowska M, Krupej, Gaus I, Zych F. Hypotensive action of prolactin in rats with spontaneous hypertension. Acta Physiol Pol 1984, 35: 215–7.
Budden R, Detweiler DK, Zbinden G. The rat electrocardiogram in pharmacology and toxicology. Oxford: Pergamon Press, 1981.
Baller D, Bretschneider HJ, Hellige G. A critical look at currently used indirect indices of myocardial oxygen consumption. Bas Res Cardiol 1981, 76: 163–70.
Manku MS, Nassar BA, Horrobin DF. Effects of prolactin on theresponses of rat aortic and arteriolar smooth-muscle preparations to noradrenaline and angiotensin. Lancet 1973, 302: 991–4.
Sowers JR, Resch G, Tempel G, Herzog J, Colantino M. Hyperprolactinaemia in the spontaneously hypertensive rat. Acta Endocrinol 1979, 90: 1–7.
Maciejewska I, Wieczorek M, Ryszka F. Wpływ prolaktyny na zmiany czynności i struktury serca po adrenalinie. Ann Acad Med Siles 1995, 29: 19–25.
Ryszka F, Dolńska B, Suszka-Świtek A. The effect of multiple doses of “Biolactin” (prolactin) on the systolic blood pressure in rats with spontaneous arterial hypertension. Pharmazie 1998, 53: 886.
Kolloch R, Kobayashi K, DeQuattro V. Dopaminergic control of sympathetic tone and blood pressure: evidence in primary hypertension. Hypertension 1980, 2: 390–4.
Nagahama S, Chen YF, Oparil S. Mechanism of the depressor effect of bromocriptine in the spontaneously hypertensive rats. J Pharmacol Exp Ther 1984, 228: 370–5.
Khorram O, Bedran de Castro JC, McCann SM. Stress-induced secretion of alpha-melanocyte-stimulating hormone and its physiological role in modulating the secretion of prolactin and luteinizing hormone in the female rat. Endocrinology 1985, 117: 2483–9.
Hill JB, Lacy ER, Nagy GM, Görcs TJ, Frawley LS. Does alphamelanocyte-stimulating hormone from the pars intermedia regulate suckling-induced prolactin release? Supportive evidence from morphological and functional studies. Endocrinology 1993, 133: 2991–7.
Khorram O, McCann SM. Interaction of alpha-melanocyte-stimulating hormone with beta-endorphin to influence anterior pituitary secretion in the female rat. Endocrinology 1986, 119: 1071–5.
McCann SM, Ono N, Khorram O, Kentroti S, Aguila C. The role of brain peptides in neuroimmunomodulation. Ann N Y Acad Sci 1987, 496: 172–8.
Khorram O, Bedran de Castro JC, McCann SM. The influence of suckling on the hypothalamic and pituitary secretion of immunoreactive alpha-melanocyte-stimulating hormone. Brain Res 1986, 398: 361–5.
Vecsernyes M, Juhasz B, Der P, et al. The administration of alphamelanocyte-stimulating hormone protects the ischemic/reperfused myocardium. Eur J Pharmacol 2003, 470: 177–83.
Juhasz B, Der P, Szondoray P, Gesztelei R, Lekli I, Bak I, et al. Adrenocorticotrope hormone fragment(4–10) attenuates the ischemia/reperfusion-induced cardiac injury in isolated rat hearts. Antioxid Redox Signal 2007, 9: 1851–61.
Aguilera G, Hyde CL, Catt KJ. Angiotensin II receptors and prolactin release in pituitary lactotrophs. Endocrinology 1982, 111: 1045–50.
Canonico PL, MacLeod RM. Angiotensin peptides stimulate phosphoinositide breakdown and prolactin release in anterior pituitary cells in culture. Endocrinology 1986, 118: 233–8.
Kubota T, Aso T. Role of angiotensin on paracrine prolactin release in the pituitary gland and its possible effects on ovarian function. Horm Res 1991, 35(Suppl 1): 13–20.
Enjalbert A, Sladeczek F, Guillon G, et al. Angiotensin II and dopamine modulate both cAMP and inositol phosphate productions in anterior pituitary cells. Involvement in prolactin secretion. J Biol Chem 1986, 261: 4071–5.
Smith JB, Smith L, Brown ER, et al. Angiotensin II rapidly increases phosphatidate-phosphoinositide synthesis and phosphoinositide hydrolysis and mobilizes intracellular calcium in cultured arterial muscle cells. Proc Natl Acad Sci USA 1984, 81: 7812–6.
Saint-Andre JP, Rohmer V, Alhenc-Gelas F, Menard J, Bigorgne JC, Corvol P. Presence of renin, angiotensinogen, and converting enzyme in human pituitary lactotroph cells and prolactin adenomas. J Clin Endocrinol Metab 1986, 63: 231–7.
Dupont AG, Van Der Niepen P, Van Steirteghem AC, Vanhaelst L. Enhanced response of plasma prolactin to metoclopramide during chronic converting enzyme inhibition. Horm Metab Res 1987, 19: 212–5.
Więcek A, Kokot F, Żukowska-Szczechowska E. The effect of long-term prazosin treatment on plasma follitropin (FSH), lutropin (LH), prolactin, estradiol and testosterone concentration in male patients with essential hypertension. Pol Arch Med Wewn 1993, 89: 125–35.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krzemiñski, T.F., Mitręga, K., Porc, M. et al. Differential action of two prolactin isoforms on ischemia-and re-perfusion-induced arrhythmias in rats in vivo . J Endocrinol Invest 34, 206–215 (2011). https://doi.org/10.1007/BF03347068
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03347068