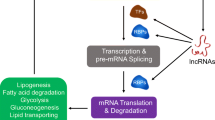
Abstract
The long non-coding RNAs (lncRNAs) are the crucial regulators of human chronic diseases. Therefore, approaches such as antisense oligonucleotides, RNAi technology, and small molecule inhibitors have been used for the therapeutic targeting of lncRNAs. During the last decade, phytochemicals and nutraceuticals have been explored for their potential against lncRNAs. The common lncRNAs known to be modulated by phytochemicals include ROR, PVT1, HOTAIR, MALAT1, H19, MEG3, PCAT29, PANDAR, NEAT1, and GAS5. The phytochemicals such as curcumin, resveratrol, sulforaphane, berberine, EGCG, and gambogic acid have been examined against lncRNAs. In some cases, formulation of phytochemicals has also been used. The disease models where phytochemicals have been demonstrated to modulate lncRNAs expression include cancer, rheumatoid arthritis, osteoarthritis, and nonalcoholic fatty liver disease. The regulation of lncRNAs by phytochemicals can affect multi-steps of tumor development. When administered in combination with the conventional drugs, phytochemicals can also produce synergistic effects on lncRNAs leading to the sensitization of cancer cells. Phytochemicals target lncRNAs either directly or indirectly by affecting a wide variety of upstream molecules. However, the potential of phytochemicals against lncRNAs has been demonstrated mostly by preclinical studies in cancer models. How the modulation of lncRNAs by phytochemicals produce therapeutic effects on cancer and other chronic diseases is discussed in this review.




Similar content being viewed by others
Abbreviations
- 3′UTR:
-
Three prime untranslated region
- AIDS:
-
Acquired immunodeficiency syndrome
- AKT:
-
AKT8 virus oncogene cellular homolog
- ALL:
-
Acute lymphoblastic leukemia
- ANRIL:
-
Antisense non-coding RNA in the INK4 locus
- ASOs:
-
Antisense oligonucleotides
- BCRP:
-
Breast cancer resistance protein
- BIK:
-
Bcl-2-interacting killer
- CAS9:
-
CRISPR-associated protein 9
- CASC2:
-
Cancer susceptibility candidate 2
- CDK6:
-
Cyclin-dependent kinase 6
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- CTR1:
-
Copper transporter 1
- DNA:
-
Deoxyribo nucleic acid
- dsDNA:
-
Double-stranded deoxyribonucleic acid
- EGCG:
-
Epigallocatechin gallate
- EIF4A3:
-
Eukaryotic translation initiation factor 4A3
- EMT:
-
Epithelial-to-mesenchymal transition
- ERα:
-
Estrogen receptor α
- FLS:
-
Fibroblast-like synoviocytes
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GAS5:
-
Growth arrest-specific 5
- GUCY2GP:
-
Guanylate cyclase 2G homolog pseudogene
- H2AFY:
-
H2A histone family member Y
- H2BFXP:
-
H2B histone family member X pseudogene
- H3K4:
-
Histone H3 lysine 4
- HFD:
-
High-fat diet
- HMGCR:
-
3-Hydroxy-3-methylglutaryl-coenzyme A reductase
- HOTAIR:
-
HOX transcript antisense intergenic RNA
- IL-6:
-
Interleukin 6
- INSIG1:
-
Insulin-induced gene 1
- JAK:
-
Janus kinase
- LINC:
-
Long intergenic non-protein-coding RNA
- linc-PINT:
-
Long intergenic non-protein-coding RNA p53 induced transcript
- LncRNA:
-
Long non-coding RNA
- MAP1LC3B2:
-
Microtubule-associated proteins 1A/1B light chain 3B
- MCP-1:
-
Monocyte chemoattractant protein-1
- MDR1/P-gp:
-
Multidrug resistance protein 1/P-glycoprotein 1
- MEG3:
-
Human maternally expressed gene 3
- MIR155HG:
-
MicroRNA155 host gene
- miRNA:
-
MicroRNA
- mRNA:
-
Messenger RNA
- MRP:
-
Multidrug resistance-associated protein
- mTOR:
-
Mammalian target of rapamycin
- NAFLD:
-
Nonalcoholic fatty liver disease
- NEAT1:
-
Nuclear paraspeckle assembly transcript 1
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NSCLC:
-
Non-small-cell lung carcinoma
- PANDAR:
-
Promoter of CDKN1A antisense DNA damage-activated RNA
- PDK4:
-
Pyruvate dehydrogenase kinase 4
- PI3K:
-
Phosphoinositide 3-kinase
- PUMA:
-
p53 up-regulated modulator of apoptosis
- PVT1:
-
Plasmacytoma variant translocation gene
- RA:
-
Rheumatoid arthritis
- RNA pol II:
-
RNA polymerase II
- RNA:
-
Ribo nucleic acid
- RNAi:
-
RNA interference
- ROR:
-
Regulator of reprogramming
- ST7OT1:
-
ST7 antisense RNA 1
- STAT:
-
Signal transducer and activator of transcription
- TGM2:
-
Transglutaminase 2
- TMEM25:
-
Transmembrane protein 25
- TNF-α:
-
Tumor necrosis factor alpha
- TNM:
-
Tumor nodes and metastasis
- TUG1:
-
Taurine-up-regulated gene 1
- TUSC7:
-
Tumor suppressor candidate 7
- Zbtb20:
-
Zinc finger and BTB domain-containing protein 20
- ZEB1:
-
Zinc-finger E-box-binding homeobox 1
- ZFAS1:
-
ZNFX1 antisense RNA 1
References
Fatica A, Bozzoni I (2014) Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 15(1):7
Hung T, Chang HY (2010) Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol 7(5):582–585
Nagano T, Fraser P (2011) No-nonsense functions for long noncoding RNAs. Cell 145(2):178–181
Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Morales DR, Thomas K, Presser A, Bernstein BE, Van Oudenaarden A (2009) Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci 106(28):11667–11672
Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP (2009) Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458(7235):223
Li Y, Wang X (2016) Role of long noncoding RNAs in malignant disease. Mol Med Rep 13(2):1463–1469
Jandura A, Krause HM (2017) The new RNA world: growing evidence for long noncoding RNA functionality. Trends Genet 33(10):665–676
Beaulieu YB, Kleinman CL, Landry-Voyer A-M, Majewski J, Bachand F (2012) Polyadenylation-dependent control of long noncoding RNA expression by the poly (A)-binding protein nuclear 1. PLoS Genet 8(11):e1003078
Guttman M, Rinn JL (2012) Modular regulatory principles of large non-coding RNAs. Nature 482(7385):339
Yin Q-F, Yang L, Zhang Y, Xiang J-F, Wu Y-W, Carmichael GG, Chen L-L (2012) Long noncoding RNAs with snoRNA ends. Mol Cell 48(2):219–230
Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, Fraser P (2008) The air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322(5908):1717–1720
Zhao J, Sun BK, Erwin JA, Song J-J, Lee JT (2008) Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322(5902):750–756
Martianov I, Ramadass A, Barros AS, Chow N, Akoulitchev A (2007) Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature 445(7128):666
Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R (2008) Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 454(7200):126
Chen L-L, Carmichael GG (2010) Decoding the function of nuclear long non-coding RNAs. Curr Opin Cell Biol 22(3):357–364
Loewer S, Cabili MN, Guttman M, Loh Y-H, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S (2010) Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet 42(12):1113
Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L (2011) lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 477(7364):295
Ng SY, Johnson R, Stanton LW (2012) Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J 31(3):522–533
Deng K, Wang H, Guo X, Xia J (2015) The cross talk between long, non-coding RNAs and microRNAs in gastric cancer. Acta Biochim Biophys Sin 48(2):111–116
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP (2011) A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146(3):353–358
Xia T, Liao Q, Jiang X, Shao Y, Xiao B, Xi Y, Guo J (2014) Long noncoding RNA associated-competing endogenous RNAs in gastric cancer. Sci Rep 4:6088
Zhuang M, Gao W, Xu J, Wang P, Shu Y (2014) The long non-coding RNA H19-derived miR-675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1. Biochem Biophys Res Commun 448(3):315–322
Leucci E, Vendramin R, Spinazzi M, Laurette P, Fiers M, Wouters J, Radaelli E, Eyckerman S, Leonelli C, Vanderheyden K, Rogiers A, Hermans E, Baatsen P, Aerts S, Amant F, Van Aelst S, van den Oord J, de Strooper B, Davidson I, Lafontaine DL, Gevaert K, Vandesompele J, Mestdagh P, Marine JC (2016) Melanoma addiction to the long non-coding RNA SAMMSON. Nature 531(7595):518–522
Anastasiadou E, Jacob LS, Slack FJ (2018) Non-coding RNA networks in cancer. Nat Rev Cancer 18(1):5–18
Fu X, Ravindranath L, Tran N, Petrovics G, Srivastava S (2006) Regulation of apoptosis by a prostate-specific and prostate cancer-associated noncoding gene, PCGEM1. DNA Cell Biol 25(3):135–141
Kurian L, Aguirre A, Sancho-Martinez I, Benner C, Hishida T, Nguyen TB, Reddy P, Nivet E, Krause MN, Nelles DA (2015) Identification of novel long non-coding RNAs underlying vertebrate cardiovascular development. Circulation 131(14):1278–1290
Casero D, Sandoval S, Seet CS, Scholes J, Zhu Y, Ha VL, Luong A, Parekh C, Crooks GM (2015) Long non-coding RNA profiling of human lymphoid progenitor cells reveals transcriptional divergence of B cell and T cell lineages. Nat Immunol 16(12):1282
Sánchez Y, Huarte M (2013) Long non-coding RNAs: challenges for diagnosis and therapies. Nucleic Acid Ther 23(1):15–20
Leucci E (2018) Cancer development and therapy resistance: spotlights on the dark side of the genome. Pharmacol Ther 189:22–30
Chandra Gupta S, Nandan Tripathi Y (2017) Potential of long non-coding RNAs in cancer patients: from biomarkers to therapeutic targets. Int J Cancer 140(9):1955–1967
Mizrahi A, Czerniak A, Levy T, Amiur S, Gallula J, Matouk I, Abu-lail R, Sorin V, Birman T, de Groot N (2009) Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences. J Transl Med 7(1):69
Bishayee A, Sethi G (2016) Bioactive natural products in cancer prevention and therapy: progress and promise. Semin Cancer Biol 40–41:1–3
Sethi G, Tergaonkar V (2009) Potential pharmacological control of the NF-kappaB pathway. Trends Pharmacol Sci 30(6):313–321
Reddy L, Odhav B, Bhoola K (2003) Natural products for cancer prevention: a global perspective. Pharmacol Ther 99(1):1–13
Block G, Patterson B, Subar A (1992) Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer 18(1):1–29
Benetou V, Orfanos P, Lagiou P, Trichopoulos D, Boffetta P, Trichopoulou A (2008) Vegetables and fruits in relation to cancer risk: evidence from the Greek EPIC cohort study. Cancer Epidemiol Prev Biomark 17(2):387–392
Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC (2008) Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer 122(10):2330–2336
Steinmetz KA, Potter JD (1996) Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc 96(10):1027–1039
Gupta SC, Kim JH, Prasad S, Aggarwal BB (2010) Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 29(3):405–434
Deorukhkar A, Krishnan S, Sethi G, Aggarwal BB (2007) Back to basics: how natural products can provide the basis for new therapeutics. Expert Opin Investig Drugs 16(11):1753–1773
Yang SF, Weng CJ, Sethi G, Hu DN (2013) Natural bioactives and phytochemicals serve in cancer treatment and prevention. Evid Based Complement Altern Med eCAM 2013:698190
Tang CH, Sethi G, Kuo PL (2014) Novel medicines and strategies in cancer treatment and prevention. Biomed Res Int 2014:474078
Hsieh YS, Yang SF, Sethi G, Hu DN (2015) Natural bioactives in cancer treatment and prevention. Biomed Res Int 2015:182835
Yarla NS, Bishayee A, Sethi G, Reddanna P, Kalle AM, Dhananjaya BL, Dowluru KS, Chintala R, Duddukuri GR (2016) Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin Cancer Biol 40–41:48–81
Hasanpourghadi M, Looi CY, Pandurangan AK, Sethi G, Wong WF, Mustafa MR (2017) Phytometabolites targeting the warburg effect in cancer cells: a mechanistic review. Curr Drug Targets 18(9):1086–1094
Shanmugam MK, Warrier S, Kumar AP, Sethi G, Arfuso F (2017) Potential role of natural compounds as anti-angiogenic agents in cancer. Curr Vasc Pharmacol 15(6):503–519
Shanmugam MK, Kannaiyan R, Sethi G (2011) Targeting cell signaling and apoptotic pathways by dietary agents: role in the prevention and treatment of cancer. Nutr Cancer 63(2):161–173
Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S (2007) Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem 102(3):580–592
Jung YY, Hwang ST, Sethi G, Fan L, Arfuso F, Ahn KS (2018) Potential anti-inflammatory and anti-cancer properties of farnesol. Molecules (Basel, Switzerland) 23(11):E2827
Merarchi M, Sethi G, Fan L, Mishra S, Arfuso F, Ahn KS (2018) Molecular targets modulated by fangchinoline in tumor cells and preclinical models. Molecules (Basel, Switzerland) 23(10):E2538
Sethi G, Shanmugam MK, Warrier S, Merarchi M, Arfuso F, Kumar AP, Bishayee A (2018) Pro-apoptotic and anti-cancer properties of diosgenin: a comprehensive and critical review. Nutrients 10(5):E645
Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS (2017) The role of resveratrol in cancer therapy. Int J Mol Sci 18(12):E2589
Tewari D, Nabavi SF, Nabavi SM, Sureda A, Farooqi AA, Atanasov AG, Vacca RA, Sethi G, Bishayee A (2018) Targeting activator protein 1 signaling pathway by bioactive natural agents: possible therapeutic strategy for cancer prevention and intervention. Pharmacol Res 128:366–375
Shanmugam MK, Lee JH, Chai EZ, Kanchi MM, Kar S, Arfuso F, Dharmarajan A, Kumar AP, Ramar PS, Looi CY, Mustafa MR, Tergaonkar V, Bishayee A, Ahn KS, Sethi G (2016) Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin Cancer Biol 40–41:35–47
Shanmugam MK, Nguyen AH, Kumar AP, Tan BK, Sethi G (2012) Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: potential role in prevention and therapy of cancer. Cancer Lett 320(2):158–170
Shrimali D, Shanmugam MK, Kumar AP, Zhang J, Tan BK, Ahn KS, Sethi G (2013) Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett 341(2):139–149
Gupta SC, Patchva S, Koh W, Aggarwal BB (2012) Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol 39(3):283–299
Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB (2011) Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep 28(12):1937–1955
Sun Y, Pan J, Zhang N, Wei W, Yu S, Ai L (2017) Knockdown of long non-coding RNA H19 inhibits multiple myeloma cell growth via NF-κB pathway. Sci Rep 7(1):18079
Awasthee N, Rai V, Verma SS, Sajin Francis K, Nair MS, Gupta SC (2018) Anti-cancer activities of Bharangin against breast cancer: evidence for the role of NF-kappaB and lncRNAs. Biochim Biophys Acta Gen Subj 1862 12:2738–2749
Zhang L, Yang F, J-H Yuan, S-X Yuan, W-p Zhou, Huo X-s XuD, H-s Bi, Wang F, S-h Sun (2012) Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma. Carcinogenesis 34(3):577–586
Hibi K, Nakamura H, Hirai A, Fujikake Y, Kasai Y, Akiyama S, Ito K, Takagi H (1996) Loss of H19 imprinting in esophageal cancer. Cancer Res 56(3):480–482
Wang S-H, Ma F, Tang Z-h WuX-C, Cai Q, Zhang M-D, Weng M-Z, Zhou D, Wang J-D, Quan Z-W (2016) Long non-coding RNA H19 regulates FOXM1 expression by competitively binding endogenous miR-342-3p in gallbladder cancer. J Exp Clin Cancer Res 35(1):160
Kondo M, Suzuki H, Ueda R, Osada H, Takagi K, Takahashi T (1995) Frequent loss of imprinting of the H19 gene is often associated with its overexpression in human lung cancers. Oncogene 10(6):1193–1198
Pan J (2017) LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur Rev Med Pharmacol Sci 21(2):322–328
Novak Kujundžić R, Grbeša I, Ivkić M, Katdare M, Gall-Trošelj K (2008) Curcumin downregulates H19 gene transcription in tumor cells. J Cell Biochem 104(5):1781–1792
Liu G, Xiang T, Wu QF, Wang WX (2016) Curcumin suppresses the proliferation of gastric cancer cells by downregulating H19. Oncol Lett 12(6):5156–5162
H-x Zhan, Wang Y, Li C, J-w Xu, Zhou B, J-k Zhu, H-f Han, Wang L, Wang Y-S, Hu S-Y (2016) LincRNA-ROR promotes invasion, metastasis and tumor growth in pancreatic cancer through activating ZEB1 pathway. Cancer Lett 374(2):261–271
Hou P, Zhao Y, Li Z, Yao R, Ma M, Gao Y, Zhao L, Zhang Y, Huang B, Lu J (2014) LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis 5(6):e1287
Wang S-H, Zhang M-D, Wu X-C, Weng M-Z, Zhou D, Quan Z-W (2016) Overexpression of LncRNA-ROR predicts a poor outcome in gallbladder cancer patients and promotes the tumor cells proliferation, migration, and invasion. Tumor Biol 37(9):12867–12875
Li L, Gu M, You B, Shi S, Shan Y, Bao L, You Y (2016) Long non-coding RNA ROR promotes proliferation, migration and chemoresistance of nasopharyngeal carcinoma. Cancer Sci 107(9):1215–1222
Chen S, Zhu J, Wang F, Guan Z, Ge Y, Yang X, Cai J (2017) LncRNAs and their role in cancer stem cells. Oncotarget 8(66):110685
Liu T, Chi H, Chen J, Chen C, Huang Y, Xi H, Xue J, Si Y (2017) Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene 631:29–38
Garitano-Trojaola A, San José-Enériz E, Ezponda T, Unfried JP, Carrasco-León A, Razquin N, Barriocanal M, Vilas-Zornoza A, Sangro B, Segura V (2018) Deregulation of linc-PINT in acute lymphoblastic leukemia is implicated in abnormal proliferation of leukemic cells. Oncotarget 9(16):12842
Marín-Béjar O, Mas AM, González J, Martinez D, Athie A, Morales X, Galduroz M, Raimondi I, Grossi E, Guo S (2017) The human lncRNA LINC-PINT inhibits tumor cell invasion through a highly conserved sequence element. Genome Biol 18(1):202
Pickard M, Williams G (2015) Molecular and cellular mechanisms of action of tumour suppressor GAS5 LncRNA. Genes 6(3):484–499
Yin D, He X, Zhang E, Kong R, De W, Zhang Z (2014) Long noncoding RNA GAS5 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Med Oncol 31(11):253
Esmatabadi MJD, Motamedrad M, Sadeghizadeh M (2018) Down-regulation of lncRNA, GAS5 decreases chemotherapeutic effect of dendrosomal curcumin (DNC) in breast cancer cells. Phytomedicine 42:56–65
Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P (2011) Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet 43(7):621
Zhan Y, Lin J, Liu Y, Chen M, Chen X, Zhuang C, Liu L, Xu W, Chen Z, He A (2016) Up-regulation of long non-coding RNA PANDAR is associated with poor prognosis and promotes tumorigenesis in bladder cancer. J Exp Clin Cancer Res 35(1):83
Ma P, Xu T, Huang M, Shu Y (2016) Increased expression of LncRNA PANDAR predicts a poor prognosis in gastric cancer. Biomed Pharmacother 78:172–176
Peng W, Fan H (2015) Long non-coding RNA PANDAR correlates with poor prognosis and promotes tumorigenesis in hepatocellular carcinoma. Biomed Pharmacother 72:113–118
Xu Y, Jiang X, Cui Y (2017) Upregulated long noncoding RNA PANDAR predicts an unfavorable prognosis and promotes tumorigenesis in cholangiocarcinoma. OncoTargets Ther 10:2873
Lu M, Liu Z, Li B, Wang G, Li D, Zhu Y (2017) The high expression of long non-coding RNA PANDAR indicates a poor prognosis for colorectal cancer and promotes metastasis by EMT pathway. J Cancer Res Clin Oncol 143(1):71–81
Chen T, Yang P, Wang H, He Z-Y (2017) Silence of long noncoding RNA PANDAR switches low-dose curcumin-induced senescence to apoptosis in colorectal cancer cells. OncoTargets Ther 10:483
Li X, Wu Z, Mei Q, Guo M, Fu X, Han W (2013) Long non-coding RNA HOTAIR, a driver of malignancy, predicts negative prognosis and exhibits oncogenic activity in oesophageal squamous cell carcinoma. Br J Cancer 109(8):2266
Pei C-S, Wu H-Y, Fan F-T, Wu Y, Shen C-S, Pan L-Q (2014) Influence of curcumin on HOTAIR-mediated migration of human renal cell carcinoma cells. Asian Pac J Cancer Prev APJCP 15(10):4239–4243
Babaei E, Sadeghizadeh M, Hassan ZM, Feizi MAH, Najafi F, Hashemi SM (2012) Dendrosomal curcumin significantly suppresses cancer cell proliferation in vitro and in vivo. Int Immunopharmacol 12(1):226–234
Mirgani MT, Isacchi B, Sadeghizadeh M, Marra F, Bilia AR, Mowla SJ, Najafi F, Babaei E (2014) Dendrosomal curcumin nanoformulation downregulates pluripotency genes via miR-145 activation in U87MG glioblastoma cells. Int J Nanomed 9:403
Zamani M, Sadeghizadeh M, Behmanesh M, Najafi F (2015) Dendrosomal curcumin increases expression of the long non-coding RNA gene MEG3 via up-regulation of epi-miRs in hepatocellular cancer. Phytomedicine 22(10):961–967
Zhou Y, Zhang X, Klibanski A (2012) MEG3 non-coding RNA: a tumor suppressor. J Mol Endocrinol 48(3):R45–R53
Rajeshkumar N, Rasheed ZA, García-García E, López-Ríos F, Fujiwara K, Matsui WH, Hidalgo M (2010) A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol Cancer Ther 9(9):2582–2592
Huang C, Yu W, Wang Q, Cui H, Wang Y, Zhang L, Han F, Huang T (2015) Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med 106(3):143–149
Bardeesy N, DePinho RA (2002) Pancreatic cancer biology and genetics. Nat Rev Cancer 2(12):897
Zhou Q, Chen J, Feng J, Wang J (2016) Long noncoding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulating hormone receptor (TSHR). Tumor Biol 37(3):3105–3113
Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ, Zhang J, Huang H (2015) LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget 6(38):41045
Zhang K, Sun X, Zhou X, Han L, Chen L, Shi Z, Zhang A, Ye M, Wang Q, Liu C (2015) Long non-coding RNA HOTAIR promotes glioblastoma cell cycle progression in an EZH2 dependent manner. Oncotarget 6(1):537
You L, Chang D, Du H-Z, Zhao Y-P (2011) Genome-wide screen identifies PVT1 as a regulator of Gemcitabine sensitivity in human pancreatic cancer cells. Biochem Biophys Res Commun 407(1):1–6
Yoshida K, Toden S, Ravindranathan P, Han H, Goel A (2017) Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis 38(10):1036–1046
Tseng Y-Y, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell TC (2014) PVT1 dependence in cancer with MYC copy-number increase. Nature 512(7512):82
Avan A, Crea F, Paolicchi E, Funel N, Galvani E, Marquez VE, Honeywell RJ, Danesi R, Peters GJ, Giovannetti E (2012) Molecular mechanisms involved in the synergistic interaction of the EZH2 inhibitor 3-deazaneplanocin A (DZNeP) with gemcitabine in pancreatic cancer cells. Mol Cancer Ther 11(8):1735–1746
Hong SP, Wen J, Bang S, Park S, Song SY (2009) CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer 125(10):2323–2331
Ottinger S, Klöppel A, Rausch V, Liu L, Kallifatidis G, Gross W, Gebhard MM, Brümmer F, Herr I (2012) Targeting of pancreatic and prostate cancer stem cell characteristics by Crambe crambe marine sponge extract. Int J Cancer 130(7):1671–1681
Sharma N, Nanta R, Sharma J, Gunewardena S, Singh KP, Shankar S, Srivastava RK (2015) PI3K/AKT/mTOR and sonic hedgehog pathways cooperate together to inhibit human pancreatic cancer stem cell characteristics and tumor growth. Oncotarget 6(31):32039
Xia P, Xu X-Y (2015) PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res 5(5):1602
Takahashi K, Yan IK, Kogure T, Haga H, Patel T (2014) Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio 4(1):458–467
Nawaz M, Fatima F, Nazarenko I, Ekström K, Murtaza I, Anees M, Sultan A, Neder L, Camussi G, Valadi H (2016) Extracellular vesicles in ovarian cancer: applications to tumor biology, immunotherapy and biomarker discovery. Expert Rev Proteomics 13(4):395–409
Yeung CLA, Tsuruga T, Yeung T-L, Kwan S-Y, Leung CS, Li Y, Lu ES, Kwan K, Wong K-K, Schmandt R (2016) Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun 7:11150
Zhang J, Liu J, Xu X, Li L (2017) Curcumin suppresses cisplatin resistance development partly via modulating extracellular vesicle-mediated transfer of MEG3 and miR-214 in ovarian cancer. Cancer Chemother Pharmacol 79(3):479–487
Wang Q, Fan H, Liu Y, Yin Z, Cai H, Liu J, Wang Z, Shao M, Sun X, Diao J (2014) Curcumin enhances the radiosensitivity in nasopharyngeal carcinoma cells involving the reversal of differentially expressed long non-coding RNAs. Int J Oncol 44(3):858–864
Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB (2011) Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci 1215(1):150–160
Borriello A, Bencivenga D, Caldarelli I, Tramontano A, Borgia A, Zappia V, Della Ragione F (2014) Resveratrol: from basic studies to bedside. In: Advances in nutrition and cancer. Springer, New York, pp 167–184
Kulkarni SS, Cantó C (2015) The molecular targets of resveratrol. Biochim Biophys Acta (BBA) Mol Basis Dis 1852(6):1114–1123
Britton RG, Kovoor C, Brown K (2015) Direct molecular targets of resveratrol: identifying key interactions to unlock complex mechanisms. Ann N Y Acad Sci 1348(1):124–133
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127(6):1109–1122
Al Aameri RF, Sheth S, Alanisi EM, Borse V, Mukherjea D, Rybak LP, Ramkumar V (2017) Tonic suppression of PCAT29 by the IL-6 signaling pathway in prostate cancer: reversal by resveratrol. PLoS One 12(5):e0177198
Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD (2011) Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol 29(8):742
Malik R, Patel L, Prensner JR, Shi Y, Iyer MK, Subramaniyan S, Carley A, Niknafs YS, Sahu A, Han S (2014) The lncRNA PCAT29 inhibits oncogenic phenotypes in prostate cancer. Mol Cancer Res 12(8):1081–1087
Yang Y, Xu H, Huang W, Ding M, Xiao J, Yang D, Li H, Liu XY, Chu L (2015) Targeting lung cancer stem-like cells with TRAIL gene armed oncolytic adenovirus. J Cell Mol Med 19(5):915–923
Gutschner T, Hämmerle M, Diederichs S (2013) MALAT1—a paradigm for long noncoding RNA function in cancer. J Mol Med 91(7):791–801
Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E (2003) MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22(39):8031
Zhang B, Arun G, Mao YS, Lazar Z, Hung G, Bhattacharjee G, Xiao X, Booth CJ, Wu J, Zhang C (2012) The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep 2(1):111–123
Nakagawa S, Ip JY, Shioi G, Tripathi V, Zong X, Hirose T, Prasanth KV (2012) Malat1 is not an essential component of nuclear speckles in mice. RNA (New York, NY) 18(8):1487–1499
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, Sun J, Cai J, Qin J, Ren J (2013) Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One 8(11):e78700
Liu Q, Sun S, Yu W, Jiang J, Zhuo F, Qiu G, Xu S, Jiang X (2015) Altered expression of long non-coding RNAs during genotoxic stress-induced cell death in human glioma cells. J Neurooncol 122(2):283–292
Fumoleau P, Seidman AD, Trudeau ME, Chevallier B, Huinink WTB (1997) Docetaxel: a new active agent in the therapy of metastatic breast cancer. Expert Opin Investig Drugs 6(12):1853–1865
de Weger VA, Beijnen JH, Schellens JH (2014) Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel–a review. Anticancer Drugs 25(5):488–494
Wani MC, Horwitz SB (2014) Nature as a Remarkable Chemist: a personal story of the discovery and development of Taxol®. Anticancer Drugs 25(5):482
Ding Y, Duan K, Chen S (2017) Low expression of lncRNA-GAS5 promotes epithelial–mesenchymal transition of breast cancer cells in vitro. J South Med Univ 37(11):1427–1435
Si X, Zang R, Zhang E, Liu Y, Shi X, Zhang E, Shao L, Li A, Yang N, Han X (2016) LncRNA H19 confers chemoresistance in ERα-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. Oncotarget 7(49):81452
Li Y, Wang Y, Wang H, Zhang L, Ding Y, Chen S, Yang Q, Chen C (2017) Effects of lncRNA RP11-770J1. 3 and TMEM25 expression on paclitaxel resistance in human breast cancer cells. J Zhejiang Univ Med Sci 46(4):364–370
Pan Y, Pan Y, Cheng Y, Yang F, Yao Z, Wang O (2018) Knockdown of LncRNA MAPT-AS1 inhibits proliferation and migration and sensitizes cancer cells to paclitaxel by regulating MAPT expression in ER-negative breast cancers. Cell Biosci 8(1):7
Guo Z, Wang Y, Zhao Y, Jin Y, An L, Wu B, Liu Z, Chen X, Zhou H, Wang H (2017) Genetic polymorphisms of long non-coding RNA GAS5 predict platinum-based concurrent chemoradiotherapy response in nasopharyngeal carcinoma patients. Oncotarget 8(37):62286
Bida O, Gidoni M, Ideses D, Efroni S, Ginsberg D (2015) A novel mitosis-associated lncRNA, MA-linc1, is required for cell cycle progression and sensitizes cancer cells to paclitaxel. Oncotarget 6(29):27880
Xu J, Wu J, Fu C, Teng F, Liu S, Dai C, Shen R, Jia X (2018) Multidrug resistant lncRNA profile in chemotherapeutic sensitive and resistant ovarian cancer cells. J Cell Physiol 233(6):5034–5043
Fang J, Qiao F, Tu J, Xu J, Ding F, Liu Y, Akuo BA, Hu J, Shao S (2017) High expression of long non-coding RNA NEAT1 indicates poor prognosis of human cancer. Oncotarget 8(28):45918
Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L, Chen S, Li Y (2014) Inhibition of long non-coding RNA NEAT1 impairs myeloid differentiation in acute promyelocytic leukemia cells. BMC Cancer 14(1):693
An J, Lv W, Zhang Y (2017) LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. OncoTargets Ther 10:5377
Tian X, Zhang H, Zhang B, Zhao J, Li T, Zhao Y (2017) Microarray expression profile of long non-coding RNAs in paclitaxel-resistant human lung adenocarcinoma cells. Oncol Rep 38(1):293–300
Liu F, Gao H, Li S, Ni X, Zhu Z (2017) Long non-coding RNA ZFAS1 correlates with clinical progression and prognosis in cancer patients. Oncotarget 8(37):61561
Hansji H, Leung EY, Baguley BC, Finlay GJ, Cameron-Smith D, Figueiredo VC, Askarian-Amiri ME (2016) ZFAS1: a long noncoding RNA associated with ribosomes in breast cancer cells. Biol Direct 11(1):62
Zhang Z, Weaver DL, Olsen D, Peng Z, Ashikaga T, Evans MF (2016) Long non-coding RNA chromogenic in situ hybridisation signal pattern correlation with breast tumour pathology. J Clin Pathol 69(1):76–81
Askarian-Amiri ME, Crawford J, French JD, Smart CE, Smith MA, Clark MB, Ru K, Mercer TR, Thompson ER, Lakhani SR, Vargas AC, Campbell IG, Brown MA, Dinger ME, Mattick JS (2011) SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA (New York, NY) 17(5):878–891
Gao K, Ji Z, She K, Yang Q, Shao L (2017) Long non-coding RNA ZFAS1 is an unfavourable prognostic factor and promotes glioma cell progression by activation of the Notch signaling pathway. Biomed Pharmacother 87:555–560
Nieto M, Huang R, Jackson R, Thiery J (2016) EMT. Cell 166(2016):21–45
Thorenoor N, Faltejskova-Vychytilova P, Hombach S, Mlcochova J, Kretz M, Svoboda M, Slaby O (2016) Long non-coding RNA ZFAS1 interacts with CDK1 and is involved in p53-dependent cell cycle control and apoptosis in colorectal cancer. Oncotarget 7(1):622
Wei Y-H, Fu Y, Luo H-J, Li R, Li H-Y, Zhang Z, Zhu Y-H, Gao Y, Liu X-L (2017) Higher expression of ZFAS1 is associated with poor prognosis in malignant melanoma and promotes cell proliferation and invasion. Int J Clin Exp Pathol 10(4):4640–4646
Ding J, Li D, Gong M, Wang J, Huang X, Wu T, Wang C (2014) Expression and clinical significance of the long non-coding RNA PVT1 in human gastric cancer. OncoTargets Ther 7:1625
Shang C, Lang B, Ao CN, Meng L (2017) Long non-coding RNA tumor suppressor candidate 7 advances chemotherapy sensitivity of endometrial carcinoma through targeted silencing of miR-23b. Tumor Biol 39(6):1010428317707883
Chen H, Xin Y, Zhou L, J-m Huang, Tao L, Cheng L, Tian J (2014) Cisplatin and paclitaxel target significant long noncoding RNAs in laryngeal squamous cell carcinoma. Med Oncol 31(11):246
Shen C-J, Cheng Y-M, Wang C-L (2017) LncRNA PVT1 epigenetically silences miR-195 and modulates EMT and chemoresistance in cervical cancer cells. J Drug Target 25(7):637–644
Wang Q, Zhang W, Hao S (2017) LncRNA CCAT1 modulates the sensitivity of paclitaxel in nasopharynx cancers cells via miR-181a/CPEB2 axis. Cell Cycle 16(8):795–801
Zhu Q-N, Wang G, Guo Y, Peng Y, Zhang R, Deng J-L, Li Z-X, Zhu Y-S (2017) LncRNA H19 is a major mediator of doxorubicin chemoresistance in breast cancer cells through a cullin4A-MDR1 pathway. Oncotarget 8(54):91990
Li W, Li H, Zhang L, Hu M, Li F, Deng J, An M, Wu S, Ma R, Lu J (2017) LINC00672 contributes p53-mediated gene suppression and promotes endometrial cancer chemosensitivity. J Biol Chem 292(14):5801–5813
Ren S, Li G, Liu C, Cai T, Su Z, Wei M, She L, Tian Y, Qiu Y, Zhang X (2016) Next generation deep sequencing identified a novel lncRNA n375709 associated with paclitaxel resistance in nasopharyngeal carcinoma. Oncol Rep 36(4):1861–1867
Jiang Y-Z, Liu Y-R, Xu X-E, Jin X, Hu X, Yu K-D, Shao Z-M (2016) Transcriptome analysis of triple-negative breast cancer reveals an integrated mRNA-lncRNA signature with predictive and prognostic value. Cancer Res 76(8):2105–2114
Chen Y-M, Liu Y, Wei H-Y, Lv K-Z, Fu P (2016) Linc-ROR induces epithelial–mesenchymal transition and contributes to drug resistance and invasion of breast cancer cells. Tumor Biol 37(8):10861–10870
Ren K, Xu R, Huang J, Zhao J, Shi W (2017) Knockdown of long non-coding RNA KCNQ1OT1 depressed chemoresistance to paclitaxel in lung adenocarcinoma. Cancer Chemother Pharmacol 80(2):243–250
Zou S, Du X, Lin H, Wang P, Li M (2018) Paclitaxel inhibits the progression of cervical cancer by inhibiting autophagy via lncRNARP11-381N20. 2. Eur Rev Med Pharmacol Sci 22(10):3010–3017
Wu D, Wang J, Pae M, Meydani SN (2012) Green tea EGCG, T cells, and T cell-mediated autoimmune diseases. Mol Aspects Med 33(1):107–118
Riegsecker S, Wiczynski D, Kaplan MJ, Ahmed S (2013) Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci 93(8):307–312
Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, Nakachi K, Fujiki H (1999) Green tea and cancer chemoprevention. Mutat Res Fundam Mol Mech Mutagen 428(1):339–344
Kuroda Y, Hara Y (1999) Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res Rev Mutat Res 436(1):69–97
Stoner GD, Mukhtar H (1995) Polyphenols as cancer chemopreventive agents. J Cell Biochem 59(S22):169–180
Tran PL, Kim S-A, Choi HS, Yoon J-H, Ahn S-G (2010) Epigallocatechin-3-gallate suppresses the expression of HSP70 and HSP90 and exhibits anti-tumor activity in vitro and in vivo. BMC Cancer 10(1):276
Yang CS (1997) Inhibition of carcinogenesis by tea. Nature 389(6647):134
Dong Z, W-y Ma, Huang C, Yang CS (1997) Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols,(–)-epigallocatechin gallate, and theaflavins. Cancer Res 57(19):4414–4419
Momose Y, Maeda-Yamamoto M, Nabetani H (2016) Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. Int J Food Sci Nutr 67(6):606–613
Mukhtar H, Ahmad N (1999) Mechanism of cancer chemopreventive activity of green tea. Proc Soc Exp Biol Med 220(4):234–238
Rahmani AH, Allemailem KS, Aly SM, Khan MA (2015) Implications of green tea and its constituents in the prevention of cancer via the modulation of cell signalling pathway. Biomed Res Int 2015:925640
Liu G, Zheng X, Xu Y, Lu J, Chen J, Huang X (2015) Long non-coding RNAs expression profile in HepG2 cells reveals the potential role of long non-coding RNAs in the cholesterol metabolism. Chin Med J 128(1):91
Gridelli C, Sacco PC (2016) Novel cytotoxic drugs in advanced nonsmall cell lung cancer. Curr Opin Oncol 28(2):110–114
Larson CA, Blair BG, Safaei R, Howell SB (2008) The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol 75(2):324–330
Tsai C-Y, Larson CA, Safaei R, Howell SB (2014) Molecular modulation of the copper and cisplatin transport function of CTR1 and its interaction with IRS-4. Biochem Pharmacol 90(4):379–387
Kalayda GV, Wagner CH, Jaehde U (2012) Relevance of copper transporter 1 for cisplatin resistance in human ovarian carcinoma cells. J Inorg Biochem 116:1–10
Kim ES, Tang X, Peterson DR, Kilari D, Chow C-W, Fujimoto J, Kalhor N, Swisher SG, Stewart DJ, Wistuba II (2014) Copper transporter CTR1 expression and tissue platinum concentration in non-small cell lung cancer. Lung Cancer 85(1):88–93
Wang X, Jiang P, Wang P, Yang CS, Wang X, Feng Q (2015) EGCG enhances cisplatin sensitivity by regulating expression of the copper and cisplatin influx transporter CTR1 in ovary cancer. PLoS One 10(4):e0125402
Liss MA, Schlicht M, Kahler A, Fitzgerald R, Thomassi T, Degueme A, Hessner M, Datta MW (2010) Characterization of soy-based changes in Wnt-frizzled signaling in prostate cancer. Cancer Genomics Proteomics 7(5):245–252
Li Y, Sarkar FH (2002) Inhibition of nuclear factor κB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res 8(7):2369–2377
Kim E-K, Kwon K-B, Song M-Y, Seo S-W, Park S-J, Ka S-O, Na L, Kim K-A, Ryu D-G, So H-S (2007) Genistein protects pancreatic β cells against cytokine-mediated toxicity. Mol Cell Endocrinol 278(1–2):18–28
Yan G-R, Yin X-F, Xiao C-L, Tan Z-L, Xu S-H, He Q-Y (2011) Identification of novel signaling components in genistein-regulated signaling pathways by quantitative phosphoproteomics. J Proteomics 75(2):695–707
Hu X-J, Xie M-Y, Kluxen FM, Diel P (2014) Genistein modulates the anti-tumor activity of cisplatin in MCF-7 breast and HT-29 colon cancer cells. Arch Toxicol 88(3):625–635
Pons DG, Nadal-Serrano M, Blanquer-Rossello MM, Sastre-Serra J, Oliver J, Roca P (2014) Genistein modulates proliferation and mitochondrial functionality in breast cancer cells depending on ERalpha/ERbeta ratio. J Cell Biochem 115(5):949–958
Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM (2009) Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer 61(5):598–606
De Souza PL, Russell PJ, Kearsley JH, Howes LG (2010) Clinical pharmacology of isoflavones and its relevance for potential prevention of prostate cancer. Nutr Rev 68(9):542–555
Chen J, Liu L, Hou R, Shao Z, Wu Y, Chen X, Zhou L (2011) Calycosin promotes proliferation of estrogen receptor-positive cells via estrogen receptors and ERK1/2 activation in vitro and in vivo. Cancer Lett 308(2):144–151
Tian J, Duan Y, Bei C, Chen J (2013) Calycosin induces apoptosis by upregulation of RASD1 in human breast cancer cells MCF-7. Horm Metab Res 45(08):593–598
Chen J, Lin C, Yong W, Ye Y, Huang Z (2015) Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells. Cell Physiol Biochem 35(2):722–728
Chiyomaru T, Fukuhara S, Saini S, Majid S, Deng G, Shahryary V, Chang I, Tanaka Y, Enokida H, Nakagawa M (2014) Long noncoding RNA HOTAIR is targeted and regulated by microRNA-141 in renal carcinoma cells. J Biol Chem 289:12550–12565
Neves R, Scheel C, Weinhold S, Honisch E, Iwaniuk KM, Trompeter H-I, Niederacher D, Wernet P, Santourlidis S, Uhrberg M (2010) Role of DNA methylation in miR-200c/141 cluster silencing in invasive breast cancer cells. BMC Res Notes 3(1):219
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai M-C, Hung T, Argani P, Rinn JL (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464(7291):1071
Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S (2011) Long non-coding RNA HOTAIR regulates Polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res 71(20):6320–6326
Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S (2013) HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene 32(13):1616
Chiyomaru T, Yamamura S, Fukuhara S, Yoshino H, Kinoshita T, Majid S, Saini S, Chang I, Tanaka Y, Enokida H (2013) Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS One 8(8):e70372
Zeng J, Sun Y, Wu K, Li L, Zhang G, Yang Z, Wang Z, Zhang D, Xue Y, Chen Y (2011) Chemopreventive and chemotherapeutic effects of intravesical silibinin against bladder cancer by acting on mitochondria. Mol Cancer Ther 10(1):104–116
Wu K, Ning Z, Zeng J, Fan J, Zhou J, Zhang T, Zhang L, Chen Y, Gao Y, Wang B (2013) Silibinin inhibits β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis via dual-blocking epithelial–mesenchymal transition and stemness. Cell Signal 25(12):2625–2633
Bosch-Barrera J, Sais E, Cañete N, Marruecos J, Cuyàs E, Izquierdo A, Porta R, Haro M, Brunet J, Pedraza S (2016) Response of brain metastasis from lung cancer patients to an oral nutraceutical product containing silibinin. Oncotarget 7(22):32006
Lu W, Lin C, King TD, Chen H, Reynolds RC, Li Y (2012) Silibinin inhibits Wnt/β-catenin signaling by suppressing Wnt co-receptor LRP6 expression in human prostate and breast cancer cells. Cell Signal 24(12):2291–2296
Kim S, Jeon M, Lee J, Han J, Oh SJ, Jung T, Nam SJ, Kil WH, Lee JE (2014) Induction of fibronectin in response to epidermal growth factor is suppressed by silibinin through the inhibition of STAT3 in triple negative breast cancer cells. Oncol Rep 32(5):2230–2236
Bhatia V, Falzon M (2015) Restoration of the anti-proliferative and anti-migratory effects of 1, 25-dihydroxyvitamin D by silibinin in vitamin D-resistant colon cancer cells. Cancer Lett 362(2):199–207
Kumar S, Raina K, Agarwal C, Agarwal R (2014) Silibinin strongly inhibits the growth kinetics of colon cancer stem cell-enriched spheroids by modulating interleukin 4/6-mediated survival signals. Oncotarget 5(13):4972
Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y (2008) Silibinin inhibits cell growth and induces apoptosis by caspase activation, down-regulating survivin and blocking EGFR–ERK activation in renal cell carcinoma. Cancer Lett 272(1):61–69
Liang L, Li L, Zeng J, Gao Y, Chen Y-L, Wang Z-Q, Wang X-Y, Chang LS, He D (2012) Inhibitory effect of silibinin on EGFR signal-induced renal cell carcinoma progression via suppression of the EGFR/MMP-9 signaling pathway. Oncol Rep 28(3):999–1005
Ramasamy K, Dwyer-Nield LD, Serkova NJ, Hasebroock KM, Tyagi A, Raina K, Singh RP, Malkinson AM, Agarwal R (2010) Silibinin prevents lung tumorigenesis in wild-type but not in iNOS−/− mice: potential of real-time micro-CT in lung cancer chemoprevention studies. Clin Cancer Res 17(4):753–761
Corominas-Faja B, Oliveras-Ferraros C, Cuyàs E, Segura-Carretero A, Joven J, Martin-Castillo B, Barrajón-Catalán E, Micol V, Bosch-Barrera J, Menendez JA (2013) Stem cell-like ALDHbright cellular states in EGFR-mutant non-small cell lung cancer: a novel mechanism of acquired resistance to erlotinib targetable with the natural polyphenol silibinin. Cell Cycle 12(21):3390–3404
Shukla SK, Dasgupta A, Mehla K, Gunda V, Vernucci E, Souchek J, Goode G, King R, Mishra A, Rai I (2015) Silibinin-mediated metabolic reprogramming attenuates pancreatic cancer-induced cachexia and tumor growth. Oncotarget 6(38):41146
Zi X, Zhang J, Agarwal R, Pollak M (2000) Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer Res 60(20):5617–5620
Polachi N, Bai G, Li T, Chu Y, Wang X, Li S, Gu N, Wu J, Li W, Zhang Y (2016) Modulatory effects of silibinin in various cell signaling pathways against liver disorders and cancer—a comprehensive review. Eur J Med Chem 123:577–595
Bhatia N, Agarwal C, Agarwal R (2001) Differential responses of skin cancer-chemopreventive agents silibinin, quercetin, and epigallocatechin 3-gallate on mitogenic signaling and cell cycle regulators in human epidermoid carcinoma A431 cells. Nutr Cancer 39(2):292–299
Singh RP, Agarwal R (2005) Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer 41(13):1969–1979
Imai-Sumida M, Chiyomaru T, Majid S, Saini S, Nip H, Dahiya R, Tanaka Y, Yamamura S (2017) Silibinin suppresses bladder cancer through down-regulation of actin cytoskeleton and PI3K/Akt signaling pathways. Oncotarget 8(54):92032
Yan T-H, Lu S-W, Huang Y-Q, Que G-B, Chen J-H, Chen Y-P, Zhang H-B, Liang X-L, Jiang J-H (2014) Upregulation of the long noncoding RNA HOTAIR predicts recurrence in stage Ta/T1 bladder cancer. Tumor Biol 35(10):10249–10257
Xue Y, Ma G, Gu D, Zhu L, Hua Q, Du M, Chu H, Tong N, Chen J, Zhang Z (2015) Genome-wide analysis of long noncoding RNA signature in human colorectal cancer. Gene 556(2):227–234
Mueller S, Schmitt M, Dekant W, Stopper H, Schlatter J, Schreier P, Lutz W (1999) Occurrence of emodin, chrysophanol and physcion in vegetables, herbs and liquors. Genotoxicity and anti-genotoxicity of the anthraquinones and of the whole plants. Food Chem Toxicol 37(5):481–491
Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J (2016) Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 12(10):580
Zhou Y, Ming J, Li Y, Du X, Deng M, He B, Zhou J, Wang G, Liu S (2018) Surfactant protein D attenuates nitric oxide-stimulated apoptosis in rat chondrocyte by suppressing p38 MAPK signaling. Biochem Biophys Res Commun 495(1):526–532
Rufino AT, Rosa SC, Judas F, Mobasheri A, Lopes MC, Mendes AF (2013) Expression and function of K (ATP) channels in normal and osteoarthritic human chondrocytes: possible role in glucose sensing. J Cell Biochem 114(8):1879–1889
Liang Z, Ren C (2018) Emodin attenuates apoptosis and inflammation induced by LPS through up-regulating lncRNA TUG1 in murine chondrogenic ATDC5 cells. Biomed Pharmacother 103:897–902
Li T, Liu Y, Xiao H, Xu G (2017) Long non-coding RNA TUG1 promotes cell proliferation and metastasis in human breast cancer. Breast Cancer 24(4):535–543
Zhang E, He X, Yin D, Han L, Qiu M, Xu T, Xia R, Xu L, Yin R, De W (2017) Increased expression of long noncoding RNA TUG1 predicts a poor prognosis of gastric cancer and regulates cell proliferation by epigenetically silencing of p57. Cell Death Dis 7(2):e2109
Sun J, Ding C, Yang Z, Liu T, Zhang X, Zhao C, Wang J (2016) The long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and promotes metastasis by affecting epithelial–mesenchymal transition. J Transl Med 14(1):42
Yan G, Wang X, Yang M, Lu L, Zhou Q (2017) Long non-coding RNA TUG1 promotes progression of oral squamous cell carcinoma through upregulating FMNL2 by sponging miR-219. Am J Cancer Res 7(9):1899
Lei H, Gao Y, Xu X (2017) LncRNA TUG1 influences papillary thyroid cancer cell proliferation, migration and EMT formation through targeting miR-145. Acta Biochim Biophys Sin 49(7):588–597
Liu Q, Liu H, Cheng H, Li Y, Li X, Zhu C (2017) Downregulation of long noncoding RNA TUG1 inhibits proliferation and induces apoptosis through the TUG1/miR-142/ZEB2 axis in bladder cancer cells. OncoTargets Ther 10:2461
Zhao L, Guo Q-L, You Q-D, Wu Z-Q, Gu H-Y (2004) Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol Pharm Bull 27(7):998–1003
Zhao K, Zhang S, Song X, Yao Y, Zhou Y, You Q, Guo Q, Lu N (2017) Gambogic acid suppresses cancer invasion and migration by inhibiting TGFβ1-induced epithelial-to-mesenchymal transition. Oncotarget 8(16):27120
Wang X, Lu N, Yang Q, Gong D, Lin C, Zhang S, Xi M, Gao Y, Wei L, Guo Q (2011) Studies on chemical modification and biology of a natural product, gambogic acid (III): determination of the essential pharmacophore for biological activity. Eur J Med Chem 46(4):1280–1290
Shahabipour F, Caraglia M, Majeed M, Derosa G, Maffioli P, Sahebkar A (2017) Naturally occurring anti-cancer agents targeting EZH2. Cancer Lett 400:325–335
Wang Y, Xiang W, Wang M, Huang T, Xiao X, Wang L, Tao D, Dong L, Zeng F, Jiang G (2014) Methyl jasmonate sensitizes human bladder cancer cells to gambogic acid-induced apoptosis through down-regulation of EZH 2 expression by miR-101. Br J Pharmacol 171(3):618–635
Wang M, Guo C, Wang L, Luo G, Huang C, Li Y, Liu D, Zeng F, Jiang G, Xiao X (2018) Long noncoding RNA GAS5 promotes bladder cancer cells apoptosis through inhibiting EZH2 transcription. Cell Death Dis 9(2):238
Paul V, Yeddanapalli LM (1954) Olefinic nature of anacardic acid from Indian cashew-nut shell liquid. Nature 174(4430):604
Brown JA, Bourke E, Eriksson LA, Kerin MJ (2016) Targeting cancer using KAT inhibitors to mimic lethal knockouts. Biochem Soc Trans 44(4):979–986
Schultz DJ, Krishna A, Vittitow SL, Alizadeh-Rad N, Muluhngwi P, Rouchka EC, Klinge CM (2018) Transcriptomic response of breast cancer cells to anacardic acid. Sci Rep 8(1):8063
Tan J, Jiang X, Yin G, He L, Liu J, Long Z, Jiang Z, Yao K (2017) Anacardic acid induces cell apoptosis of prostatic cancer through autophagy by ER stress/DAPK3/Akt signaling pathway. Oncol Rep 38(3):1373–1382
Chang W (2017) Non-coding RNAs and berberine: a new mechanism of its anti-diabetic activities. Eur J Pharmacol 795:8–12
Jeong Y, You D, Kang H-G, Yu J, Kim SW, Nam SJ, Lee JE, Kim S (2018) Berberine suppresses fibronectin expression through inhibition of c-Jun phosphorylation in breast cancer cells. J Breast Cancer 21(1):21–27
Liu D, Zhang Y, Liu Y, Hou L, Li S, Tian H, Zhao T (2018) Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp Clin Endocrinol Diabetes 126(8):513–520
Lin Y, Sheng M, Ding Y, Zhang N, Song Y, Du H, Lu N, Yu W (2018) Berberine protects renal tubular cells against hypoxia/reoxygenation injury via the Sirt1/p53 pathway. J Nat Med 72(3):715–723
Nonaka M, Murata Y, Takano R, Han Y, Kabir MHB, Kato K (2018) Screening of a library of traditional Chinese medicines to identify anti-malarial compounds and extracts. Malaria J 17(1):244
Zhu X, Sun Y, Zhang C, Liu H (2017) Effects of berberine on a rat model of chronic stress and depression via gastrointestinal tract pathology and gastrointestinal flora profile assays. Mol Med Rep 15(5):3161–3171
Yuan X, Wang J, Tang X, Li Y, Xia P, Gao X (2015) Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J Transl Med 13(1):24
Hollman PC, Gaag MV, Mengelers MJ, Van Trijp JM, De Vries JH, Katan MB (1996) Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic Biol Med 21(5):703–707
Choi J-A, Kim J-Y, Lee J-Y, Kang C-M, Kwon H-J, Yoo Y-D, Kim T-W, Lee Y-S, Lee S-J (2001) Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol 19(4):837–844
Coskun O, Kanter M, Korkmaz A, Oter S (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res 51(2):117–123
Natarajan V, Krithica N, Madhan B, Sehgal PK (2011) Formulation and evaluation of quercetin polycaprolactone microspheres for the treatment of rheumatoid arthritis. J Pharm Sci 100(1):195–205
Mamani-Matsuda M, Kauss T, Al-Kharrat A, Jm Rambert, Fawaz F, Thiolat D, Moynet D, Coves S, Malvy D, Mossalayi MD (2006) Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decreased macrophage inflammatory mediators. Biochem Pharmacol 72(10):1304–1310
Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N (2001) Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Immunol 167(9):5381–5385
Gabriel SE, Michaud K (2009) Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther 11(3):229
Kurowska M, Rudnicka W, Kontny E, Janicka I, Chorazy M, Kowalczewski J, Ziółkowska M, Ferrari-Lacraz S, Strom TB, Maśliński W (2002) Fibroblast-like synoviocytes from rheumatoid arthritis patients express functional IL-15 receptor complex: endogenous IL-15 in autocrine fashion enhances cell proliferation and expression of Bcl-xL and Bcl-2. J Immunol 169(4):1760–1767
Chen S, Yang Y, Feng H, Wang H, Zhao R, Liu H (2014) Baicalein inhibits interleukin-1β-induced proliferation of human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation 37(1):163–169
Pan F, Zhu L, Lv H, Pei C (2016) Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA MALAT1. Int J Mol Med 38(5):1507–1514
Slaninová I, Pěnčíková K, Urbanová J, Slanina J, Táborská E (2014) Antitumour activities of sanguinarine and related alkaloids. Phytochem Rev 13(1):51–68
Wei G, Xu Y, Peng T, Yan J, Wang Z, Sun Z (2017) Sanguinarine exhibits antitumor activity via up-regulation of Fas-associated factor 1 in non-small cell lung cancer. J Biochem Mol Toxicol 31(8):e21914
Ma Y, Yu W, Shrivastava A, Alemi F, Lankachandra K, Srivastava RK, Shankar S (2017) Sanguinarine inhibits pancreatic cancer stem cell characteristics by inducing oxidative stress and suppressing sonic hedgehog-Gli-Nanog pathway. Carcinogenesis 38(10):1047–1056
Zhang R, Wang G, Zhang PF, Zhang J, Huang YX, Lu YM, Da W, Sun Q, Zhu JS (2017) Sanguinarine inhibits growth and invasion of gastric cancer cells via regulation of the DUSP4/ERK pathway. J Cell Mol Med 21(6):1117–1127
Kalogris C, Garulli C, Pietrella L, Gambini V, Pucciarelli S, Lucci C, Tilio M, Zabaleta ME, Bartolacci C, Andreani C (2014) Sanguinarine suppresses basal-like breast cancer growth through dihydrofolate reductase inhibition. Biochem Pharmacol 90(3):226–234
Croaker A, King GJ, Pyne JH, Anoopkumar-Dukie S, Simanek V, Liu L (2017) Carcinogenic potential of sanguinarine, a phytochemical used in ‘therapeutic’ black salve and mouthwash. Mutat Res Rev Mutat Res 774:46–56
Zhang S, Leng T, Zhang Q, Zhao Q, Nie X, Yang L (2018) Sanguinarine inhibits epithelial ovarian cancer development via regulating long non-coding RNA CASC2–EIF4A3 axis and/or inhibiting NF-κB signaling or PI3K/AKT/mTOR pathway. Biomed Pharmacother 102:302–308
Li P, Xue W-J, Feng Y, Mao Q-S (2016) Long non-coding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res 8(8):3522
He X, Liu Z, Su J, Yang J, Yin D, Han L, De W, Guo R (2016) Low expression of long noncoding RNA CASC2 indicates a poor prognosis and regulates cell proliferation in non-small cell lung cancer. Tumor Biol 37(7):9503–9510
Wang R, Li Y, Zhu G, Tian B, Zeng W, Yang Y, Li Z (2017) long noncoding rNa casc2 predicts the prognosis of glioma patients and functions as a suppressor for gliomas by suppressing Wnt/β-catenin signaling pathway. Neuropsychiatr Dis Treat 13:1805
Pei Z, Du X, Song Y, Fan L, Li F, Gao Y, Wu R, Chen Y, Li W, Zhou H (2017) Down-regulation of lncRNA CASC2 promotes cell proliferation and metastasis of bladder cancer by activation of the Wnt/β-catenin signaling pathway. Oncotarget 8(11):18145
Huang G, Wu X, Li S, Xu X, Zhu H, Chen X (2016) The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci Rep 6:26524
Zhou J, Huang H, Tong S, Huo R (2017) Overexpression of long non-coding RNA cancer susceptibility 2 inhibits cell invasion and angiogenesis in gastric cancer. Mol Med Rep 16(4):5235–5240
Baldinu P, Cossu A, Manca A, Satta MP, Sini MC, Rozzo C, Dessole S, Cherchi P, Gianfrancesco F, Pintus A (2004) Identification of a novel candidate gene, CASC2, in a region of common allelic loss at chromosome 10q26 in human endometrial cancer. Hum Mutat 23(4):318–326
Baldinu P, Cossu A, Manca A, Satta MP, Sini MC, Palomba G, Dessole S, Cherchi P, Mara L, Tanda F (2007) CASC2a gene is down-regulated in endometrial cancer. Anticancer Res 27(1A):235–243
Zhang Y, Talalay P, Cho C-G, Posner GH (1992) A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci 89(6):2399–2403
Beaver LM, Kuintzle R, Buchanan A, Wiley MW, Glasser ST, Wong CP, Johnson GS, Chang JH, Löhr CV, Williams DE (2017) Long noncoding RNAs and sulforaphane: a target for chemoprevention and suppression of prostate cancer. J Nutr Biochem 42:72–83
Fang Y, Huang Z, Li H, Tan W, Zhang Q, Wang L, Wu J (2018) LINC01116 promotes the progression of epithelial ovarian cancer via regulating cell apoptosis. Eur Rev Med Pharmacol Sci 22(16):5127–5133
Sankaram A, Rao G (1978) Bharangin, a novel diterpenoid quinonemethide from Pygmacopremna herbaceae. In: Proceedings of IUPAC 11th international symposium of chemistry of natural products, vol 1978, pp 97–100
Sankaram AVB, Marthanda Murthi M, Bhaskaraiah K, Narsimha Rao GL, Subramanyam M, Shoolery J (1988) Bharangin, a novel diterpenoid quinonemethide from Pigmacopremna herbaceae (Roxb.) Moldenke. Tetrahedron Lett 29(2):245–248
Sathish T, Brahmaiah P, Sathya K, Bhojaraju P, Naik NG, Kezia D, Prakasam RS (2009) A novel RP-HPLC method for the determination of bharangin in Ghantu bharangi crude extracts. Pak J Pharm Sci 22(1):68–73
Kirtikar KR, Basu BD (1918) Indian medicinal plants, vol 3. Lalit Mohan Basu, Allahabad, pp 883–884
Narayanan N, Thirugnanasambantham P, Viswanathan S, Reddy MK, Vijayasekaran V, Sukumar E (2000) Antipyretic, antinociceptive and anti-inflammatory activity of Premna herbacea roots. Fitoterapia 71(2):147–153
Nayar RC, Yoganarsimhan SN, Subramanyam K (1976) Pharmacognosy of a local market sample of bharangin: Pygmacopremna herbaceae. Indian J Pharm 38:39–44
Boonyaratanakornkit L, Chantaptavan V (1993) Identification and specification of khao-yen-neua and khao-yen-tai. Thai J Pharm Sci 1:79–90
Itharat A, Singchangchai P, Ratanasuwan P (1998) Wisdom of Southern Thai traditional doctors, vol 126. Prince of Songkla University, Songkla
Ravishankar K, Pai K, Isha D, Setty M, Manjula S, Ramalingayya G (2008) An appraisal of the antitumor activity of alcoholic extract of Premna herbacea Roxbin Ehrlich’s ascitic carcinoma model. Indian J Pharmacol 40(Suppl 2):67
Gupta SC, Kannappan R, Kim J, Rahman GM, Francis SK, Raveendran R, Nair MS, Das J, Aggarwal BB (2011) Bharangin, a diterpenoid quinonemethide, abolishes constitutive and inducible nuclear factor-κB (NF-κB) activation by modifying p65 on cysteine 38 residue and reducing inhibitor of nuclear factor-κB α kinase activation, leading to suppression of NF-κB-regulated gene expression and sensitization of tumor cells to chemotherapeutic agents. Mol Pharmacol 80(5):769–781
Acknowledgements
The authors would like to thank Richard Heather and Pokhrel Arya from UNMC High School Alliance Program at the University of Nebraska Medical Center, USA, for thoroughly reading the article. SCG is thankful to the Science and Engineering Research Board (ECR/2016/000034) and University Grants Commission [No.F. 30-112/2015 (BSR)] for the financial assistance. Dr. Challagundla’s laboratory is supported in whole or part from the NIH/NCI Grant (K22CA197074-01); the Nebraska State DHHS (LB506); IDeA Award from the NIH/NIGMS (P30 GM106397); UNMC Pediatric Cancer Research Center; Fred and Pamela Buffett Cancer Center’s Pilot Grant (P30 CA036727) in conjunction with the UNMC Pediatric Cancer Research Center; Leukemia Research Foundation Grant and the Department of Biochemistry and Molecular Biology start-up at UNMC. SM, SSV, and NA are supported from ICMR New Delhi (3/1/3/JRF-2016/LS/HRD-65-80388), DBT New Delhi (DBT/2017/BHU/786), and BHU Varanasi (R/Dev/IX-Sch-BHU Res Sch 2018-19), respectively.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mishra, S., Verma, S.S., Rai, V. et al. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell. Mol. Life Sci. 76, 1947–1966 (2019). https://doi.org/10.1007/s00018-019-03053-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03053-0