Abstract
The patterns in dissolved organic carbon (DOC) photo-mineralization along the freshwater continuum from land to sea are poorly known. Specifically, it has not been resolved how the photo-degradation of DOC into CO2 (PD) depends on the combination of intrinsic properties of DOC and extrinsic variables that affect the photo-reactions. We measured PD per unit of absorbed ultraviolet light energy (PD-Ew) in headwater streams, lakes, intermediate rivers and river mouths in Sweden. Surprisingly, no trend of decreasing PD-Ew was found with decreases in colored DOC. However, there was a relationship between PD-Ew and pH, best described by a quadratic (U-shaped) curve, indicating environmental control of photo-reactivity. Interestingly, the highest values for both of these variables were recorded for river mouths. Moreover, PD-Ew increased with proxy variables for the amount of autochthonous DOC in the water. Thus, changes in pH and autochthonous DOC input along the continuum may sustain high DOC photo-mineralization throughout continental aquatic networks.
Similar content being viewed by others
Introduction
Inland waters play a substantial role in generating greenhouse gas emissions to the atmosphere, in part owing to organic carbon mineralization processes (Panneer Selvam et al. 2014; Cole et al. 2007; Tranvik et al. 2009). In freshwaters, dissolved organic carbon (DOC) is subject to both bio- and photo-mineralization, although the latter has received less attention by the research community. The photochemical oxidation of DOC induced by ultraviolet (UV) sunlight irradiation produces carbon dioxide (CO2), low molecular weight organic acids, partially oxidized recalcitrant DOC, and small amounts of carbon monoxide (Cory et al. 2014; Zafiriou et al. 1984; Cory et al. 2010). It has been estimated that up to 10% of present greenhouse gas emissions from lakes globally may be generated by photo-mineralization of terrestrially-derived DOC into CO2 (Koehler et al. 2014). However, to predict how these emissions may be affected by future increasing loads of terrestrial DOC to inland waters, a better understanding of the controls on photo-mineralization rates along the aquatic continuum is needed.
The relative role of bio- vs. photo-mineralization may vary greatly across seasons and geographic location of aquatic systems. For example, the contribution from photo-mineralization to the total DOC processing during summer was less than 3% in the water column of a subtropical lagoon (Ziegler and Benner 2000), but 12% in epilimnia of boreal lakes (Granéli et al. 1996) and as much as 70–95% in arctic lakes and rivers (Cory et al. 2014). Seasonally, Vachon et al. (2016) found that the proportion of lake pelagic CO2 produced due to photo-mineralization was higher in spring compared to in summer. There are indications that dark environments such as soils, ice-covered lakes, or deep hypolimnetic water accumulate photo-sensitive DOC compounds that can later be rapidly degraded upon light exposure if mobilized (Gonsior et al. 2013). Theoretically, DOC photo-mineralization depends on a combination of intrinsic factors (presence of photo-sensitive DOC) and extrinsic variables (e.g. ions in the water affecting light absorption and radical formation). However, there is no consensus on the main causes of the variability in DOC photo-mineralization in nature. Most studies so far have focused on DOC properties, but the rates of photo-mineralization may largely be controlled by combinations of intrinsic photochemical reactivity of the DOC and extrinsic water chemistry variables (Pace et al. 2011; Porcal et al. 2014), as well as spatial and temporal variations in irradiation intensity (Vachon et al. 2016; Cory et al. 2014).
It is often assumed that the higher the aromaticity and degree of pigmentation of DOC, the more absorption of UV irradiation and thus the higher CO2 emissions caused by photo-mineralization. Support of this assumption has to some extent been found in comparisons between lakes with different color (Molot and Dillon 1997; Koehler et al. 2016). High molecular weight substances such as humic and fulvic acids are colored (Mostofa et al. 2013) and hence susceptible to photo-mineralization (Benner and Kaiser 2011). At the landscape level, color and humic DOC fractions generally decrease along the aquatic continuum (Weyhenmeyer et al. 2012), which might suggest corresponding decreases in photo-mineralization from land to sea. However, such an interpretation is not straightforward, e.g. demonstrated by Koehler et al. (2014) who found that the total light-induced DOC mineralization in the whole water column of clear and brown freshwaters was roughly the same.
Moreover, differences in photo-mineralization have been observed between systems independent of the light absorbing properties of DOC, pointing to a relatively larger importance of extrinsic factors. For example, Cory et al. (2013) found that the specific UV absorbance at 254 nm (SUVA254), which is proportional to contents of aromatic colored DOC, was not clearly related to total ecosystem-level absorption of UV irradiation by colored DOC or subsequent light-induced CO2 production. Reche et al. (1999) found that increase in alkalinity was related to higher UV light absorption by colored DOC and Pace et al. (2011) noted that increases in pH per se enhances both UV irradiation absorption by colored DOC and subsequent photochemical reactions. Additionally, nitrate and nitrite affect photo-mineralization positively by catalyzing hydroxyl radical formation due to UV light, although the concentration of nitrite is low compared to nitrate in freshwaters (Zepp et al. 1987; Porcal et al. 2014). Finally, Porcal et al. (2014) is an example of several studies showing that DOC photo-mineralization can be particularly high when iron concentrations are high, especially in combination with extremely low pH. Hence, better understanding of both intrinsic and extrinsic factors driving the DOC photo-mineralization will favor improved predictions of the fate of DOC as it transits continental watersheds with contrasting environmental conditions.
In this context, the aim of the study was to determine the variation in photo-reactivity (photo-mineralization per unit absorbed light) with decrease in color and humic substances along a gradient of naturally changing extrinsic variables (pH, iron, nitrates) and organic matter loading in the aquatic continuum from land to sea. We explored the regulation of this photo-reactivity by both intrinsic and extrinsic factors. This was done by performing photo-decay experiments with water from different aquatic systems in different regions of Sweden. In addition, we performed one-year biological incubations on selected samples to test the long-term effect of microbial degradation on the DOC photo-mineralization potential.
Materials and methods
Study site
In order to study wide ranges in variability of extrinsic variables and color, water samples were collected from headwater streams (n = 34), lakes (n = 23), intermediate rivers (n = 4) and river mouths (n = 10) located in Sweden (Fig. 1). Water samples from headwater streams were collected during 2012 (spring, summer and autumn) and 2013 (spring). The headwater streams are located near Skogaryd (58°22′N, 12°9′E) and Umeå (64°14′N, 19°46′E) and their catchments are either dominated by forest or a mix between forest and peatland. Lake water samples were collected from the epilimnion (1 m below the surface of water) near Umeå (64°14′N, 19°46′E) during spring, summer and fall 2012. Water samples from intermediate rivers were collected during 2012 (spring) from Skogaryd (58°22′N, 12°9′E) and Abisko (68°20′N, 18°58′E). River mouth samples were collected from the outlet of 10 Swedish rivers during summer 2013 at approximately 30 cm depth. The rivers are located between latitudes 55°N and 65°N latitudes along a 1300 km north–south gradient. These rivers drain catchments with mountains, forests and wetlands in the north and catchments with significant fractions of agricultural land and urban areas in the south. Samples were refrigerated during transport and stored close to 1 °C until further analysis. Samples were analyzed for DOC using Shimadzu total organic carbon analyzer 5000 (Kyoto, Japan), pH was measured in the laboratory using a Mettler Toledo DGi117-water or Mettler Delta 340, dissolved iron (Fe) was measured in filtered and acidified samples using ICP-MS and ICP-AES analysis, and finally, nitrates (NO3 + NO2) were measured using a HACH-IL 550 TOC-TN.
Long term incubation experiment
To determine the effect of increases in water residence time on photo-mineralization, the impact of microbial processing of DOC on photoreactivity and interactions between photo-mineralization and intrinsic DOC properties, we performed a long-term microbial degradation experiment in which the DOC was gradually degraded and transformed while variability of extrinsic variables was low. In these experiments 500 ml of samples from lake and headwater streams were incubated in 1000 ml Duran glass bottles at 20 °C under dark condition for 1 year. The bottles were opened every two months to oxygenate the water. The DOC concentration, optical properties and photo-reactivity were analyzed at the beginning and at end of the incubation.
Optical DOC characterization
The DOC absorbance was measured from 200 to 800 nm using a Shimadzu UV-2600 UV–VIS spectrophotometer. Later, decadic absorbance coefficient (α) (Hu et al. 2002) was converted from cm−1 to m−1 by dividing the path length in meters. Using a Cary Eclipse Fluorescence Spectrophotometer, excitation was performed from 230 to 450 nm by 5 nm increments and emission was measured from 260 to 600 nm by 2 nm increments to generate excitation emission matrices (EEMs). The EEMs were corrected for instrument specific biases and inner filter effect, and were normalized to the Raman water peak using FDOM correct toolbox for MATLAB (Murphy et al. 2010). The freshness index (FRESH; an indicator of freshness of the DOC) (Parlanti et al. 2000), fluorescence index (FI, an indicator of the origin of fulvic acids) (McKnight et al. 2001) and humification index (HIX, a humic substance indicator) (Zsolnay et al. 1999) were calculated from the corrected dataset. Further, we calculated specific absorbance at 254 nm (SUVA254, L mg C−1 m−1, an index of the intensity of absorbance by unit DOC) by dividing the absorbance at 254 nm by DOC (Weishaar et al. 2003). Colored dissolved organic matter (CDOM, m−1, Naperian units, an indicator of the concentration of terrestrial, colored DOC) was calculated by dividing the absorbance at 440 nm by path length of the cuvette in meters and multiplied by 2.303 (Cuthbert and del Giorgio 1992). Finally the ratio of absorption at 254 nm and 365 nm, respectively (a254/a365) was used as an indicator of decreasing molecular weight of the DOC (Ågren et al. 2008).
Photochemical reactivity
The filtered water samples for photochemical reactivity measurements were incubated under UV irradiation (3.64–6.89 Wm−2 for UV-A and 0.06–0.1 Wm−2 for UV-B) for 48 h. More details of the experiment are available elsewhere (Panneer Selvam et al. 2016). Briefly, 10 ml aliquots of the filtered water sample were filled in 20 ml quartz vials and the remaining 10 ml (headspace) was filled with a synthetic air mixture. For each sample, two vials were kept as control and acidified immediately with 0.1 ml of concentrated H3PO4 and two additional vials were incubated at 20 ± 1 °C under UV irradiation for 48 h. After irradiation, samples were acidified with 0.1 ml of concentrated H3PO4 to convert the dissolved inorganic carbon (DIC) in the vials to CO2, shaken for a minute, and then left for a day to attain equilibrium. After equilibrium, 5 ml of the air samples were injected into EGM-4 infrared gas analyzer (PP systems, Amesbury, MA, USA) to measure the CO2 in headspace, and the DIC was calculated by accounting for the partitioning of CO2 between the headspace and liquid phase of the sample. The DIC produced during incubation was calculated as the difference between the DIC observed after incubation and DIC from the control vials.
The photo-degradation of DOC per unit of absorbed light energy, PD-Ew, was calculated to determine the photo-degradation rate (PD) relative to the total irradiated energy absorbed by the study system (Ew). Most previous studies have focused on the absolute quantity of DOC degraded by the UV irradiation (which increases with optical density), but PD-Ew is a relevant variable since clear-water and brown-water sites in nature absorb a similar amount of total light over the whole water column (Koehler et al. 2014). Since PD-Ew is not affected by the optical density per se (absolute color), it is an important parameter for modeling the CO2 emissions caused by the total light absorption in the water column. Given that high energy-low wavelength photons are more reactive than low energy-high wavelength photons, we standardized PD to the total quantity of absorbed energy following Bertilsson and Tranvik (2000). The calculation has been explained in detail elsewhere (Bertilsson and Tranvik 2000; Panneer Selvam et al. 2016). Briefly, the calculation was based on the total absorbed energy (Ew) (Bertilsson and Tranvik 2000) of each sample between 300 and 550 nm where the major photo-mineralization process occurs (Vahatalo et al. 2000).
The Ew (J m−2) was calculated based on the lamp energy in Wm−2 (I0λ), incubation time in seconds (TUV) and the average absorption of irradiation in the quartz vials at a specific wavelength ([1–10−α]λ). In order to account for the impact of self-shading within the quartz vials, we horizontally divided the quartz vial into ten different sections (see details in Panneer Selvam et al. 2016) and calculated the mean irradiation path length of different section of the vial (L, cm) at each wavelength (Eq. 2).
It should be noted that the mean vertical pathlength in our horizontally-placed sample vials was short (0.81 cm) and does not represent the total depth of the water column that would be irradiated in nature. Thus our photo-reactivity variable PD-Ew informs quantitatively about the photo-reactivity per unit of total light absorbed in 0.81 cm depth intervals of the water column in nature.
Statistical analyses
One-way analysis of variance (ANOVA) and Tukey posthoc test was used to analyze the differences between the different optical properties and PD-Ew in the different aquatic ecosystems. In the case of long term incubation, we used paired t test to determine if there was a significant difference in DOC composition and DOC photoreactivity between the start and end of the incubation. These statistical tests were performed in IBM SPSS 22 (IBM Corp., Armonk, NY, USA).
Results
In the different types of freshwaters that we sampled along the aquatic continuum, the CDOM decreased strongly from lakes to intermediate rivers and to river mouths (Fig. S1), but there was no significant change from headwater streams to lakes. Further, these different aquatic ecosystems showed systematic and statistically different intrinsic properties of DOC (see Fig. S1 and Table S1). Most notable, river mouths had higher a254/a365 and lower SUVA254 than all the other sampled aquatic ecosystems, suggesting that a colored fraction of DOC was preferentially lost during downstream transport. River mouths also had higher FRESH than headwater streams and lakes. With regard to changes from headwaters to lakes, streams from forest had higher a254/a365 than lakes. The FRESH and a254/a365 decreased from headwater streams to lakes, and increased from intermediate rivers to river mouths (Fig. S1). Regarding the extrinsic environmental controls, river mouths had higher pH and NO3 + NO2 than the headwater streams and lakes while Fe showed no clear difference between any of the aquatic ecosystems.
The long-term incubation conducted to investigate interactions between photo-mineralization and intrinsic DOC properties showed that the a254/a365, CDOM and FI decreased, while SUVA254 and, surprisingly, FRESH increased over time (Fig. 2, Table S2).
Relative changes from the start to the end of incubation in DOC optical properties (a354/a365, FI, CDOM, FRESH [n = 38], SUVA254 [n = 35]) and in the DOC degradation rate per unit irradiation energy absorbed (PD-Ew; n = 38). Outliers were removed for clarity of the plot. The symbol asterisk denotes significant (2-tail p < 0.05) difference from start to the end of incubation
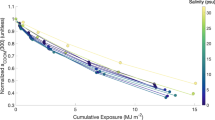
In the field data, river mouths and forest headwater streams showed no significant difference in PD-Ew but river mouths showed higher PD-Ew than the peat headwater streams, lakes and intermediate rivers (Fig. 3), demonstrating higher DOC photo-reactivity. Further, forest headwater streams had higher PD-Ew than the lakes. In the long term incubation data, the PD-Ew did not differ between the start and at the end of the incubation (Fig. 2, Table S2). Therefore, in ambient as well as in experimental conditions, there appear to be mechanisms that replenish a photo-reactive DOC pool.
The DOC degradation rate per unit irradiation energy absorbed (PD-Ew) variation in mire headwater streams (n = 8), forest headwater streams (n = 20), mixture of forest and mire headwater streams (n = 6), lakes (n = 23), intermediate rivers (n = 4) and river mouths (n = 10). Outliers were removed for clarity of the plot. Different letters indicate significant (2-tail p < 0.05) difference between the different aquatic ecosystems
When combining all field data, PD-Ew was positively correlated with absorption ratio, FRESH and FI, but weakly and negatively correlated with SUVA254 and CDOM (Fig. 4); all of these trends are in the opposite direction than what would be expected if there was intrinsic control of the photo-reactivity. In the case of extrinsic variables, pH showed U shaped relationship with PD-Ew as indicated by the polynomial curve (Fig. 4f), Fe was negatively correlated with PD-Ew but NO3 + NO2 were positively correlated with PD-Ew (Fig. 4g, h).
Pearson correlation and polynomial equation between different optical properties and PD-Ew. Data from all aquatic ecosystems are combined for each plot. a Correlation between a254/a365 and PD-Ew (n = 84), b correlation between FI and PD-Ew (n = 84), c correlation between FRESH and PD-Ew (n = 84), d correlation between CDOM and PD-Ew (n = 84), e correlation between SUVA254 and PD-Ew (n = 84), f polynomial equation of pH and PD-Ew (n = 38), g correlation between Fe and PD-Ew (n = 35) and h correlation between NO3 + NO2 and PD-Ew (n = 27)
Discussion
Terrestrial lateral export of DOC is increasing with global warming (IPCC 2013; Frey and Smith 2005, Frey and McClelland 2009), and mineralization processes in inland waters play a key role for determining the degree by which this carbon is lost to the atmosphere rather than exported to coastal regions. We found that river mouths, which represent the extreme end-members in terms of land–water connectivity, had higher DOC photo-reactivity than most of the other aquatic ecosystems studied in the boreal landscape (Fig. 3). This contrasts some previous results which have shown that the DOC photo-reactivity tends to decrease as DOC is lost with increasing water residence time because of the increase in DOC exposure to sunlight and preferential loss of the aromatic, photo-reactive fractions of DOC (Molot and Dillon 1997; Koehler et al. 2016).
It has been suggested that the rate of DOC photo-mineralization is proportional to CDOM and SUVA254, based on the reasoning that DOC first has to efficiently absorb light in order to be degraded (Benner and Kaiser 2011; Lapierre and del Giorgio 2014). However, recent empirical evidence demonstrates that the photo-mineralization of DOC can to various degrees be correlated to CDOM or to SUVA254 in natural environments, with relationships ranging from weak or absent (Cory et al. 2013) to relatively strong (Koehler et al. 2016). Our results further show that the photo-mineralization of DOC can in fact be weakly negatively correlated with CDOM and SUVA254 along our gradient of changing environmental conditions in the aquatic continuum (Fig. 4). Thus the intrinsic DOC properties that govern its color and aromaticity may influence the patterns DOC photo-reactivity to variable degrees across studies, but other (extrinsic) factors can be equally important.
It is well known that a range of extrinsic variables affect the photo-reactivity, and that classification of DOC as photo-reactive or as photo-refractory is problematic. For example, Gonsior et al. (2014) showed that dissolved organic matter fractions which have traditionally been classified as photo-refractory in fact can be potentially photo-reactive if the circumstances allow, evidenced by their reactivity when exposed directly to reactive oxygen species. In this regard, the positive correlation of PD-Ew and a254/a365 in this study suggests that low molecular weight substances may also be photo-reactive under certain environmental conditions. This is opposite to the pattern found by Koehler et al. (2016) in their study of lakes only, where DOC with high spectral slopes had low photo-reactivity. In our study, we observed higher PD-Ew in forest headwater streams (with high a254/a365) than in lakes (with low a254/a365), and higher PD-Ew in river mouths (with high a254/a365) than in most of the other aquatic ecosystems. Further, the freshness index (FRESH), which indicates recently produced DOC of plant or microbial origin, was significantly higher in river mouths than in headwater streams and lakes (Fig. S1). Possibly, the DOC in river mouths might not have an exclusive upstream origin, but could also be formed by phytoplankton or other algae during photosynthesis in river mouths, or from the breakdown of larger molecules into smaller molecules throughout the aquatic continuum (Creed et al. 2015).
A core result of this study is that extrinsic factors exert control on the broad scale patterns in the photo-mineralization of DOC, and our results suggest that pH may be one such factor (Fig. 4f–h). The CDOM comprises double bond elements like carbon, nitrogen and oxygen, and these double bonds get stronger at low pH (Gennings et al. 2001; Larson and Weber 1994) because of the more stable and compact form of the molecules in their protonated stage, leading to more efficient absorption of the most energy rich low-end wavelength radiation. This likely explains why Anesio and Granéli (2003) found relatively higher photo-mineralization at pH descending below 4. There is also a well-known interaction effect between low pH and Fe that increases the photo-reactivity (Gu et al. 2017). However, Pace et al. (2011) found higher photo-bleaching rates at alkaline conditions, compared to neutral pH, because the size of polymers and colloids dramatically expands, hence the DOC interacts with a larger amount of UV irradiation at a maximum level of deprotonation. In our study, pH ranges from 3.7 to 7.6 and speculatively we appear to capture both effects, i.e. photo-reactivity increase in both ends of the pH scale, resulting in a U-shaped relationship between pH and PD-Ew (Fig. 4f). This quadratic curve explains roughly twice as much variability as a corresponding linear or exponential curve, signifying that the U-shaped curve has significantly better fit, but it can be noted that the relationship is not symmetrical as the decrease in photo-reactivity from 3.7 to ca 6.5 is relatively flat compared to the steeper increase occurring around a pH of 7.
The oxidation by short-lived and highly reactive hydroxyl radicals plays an important role in the photo-mineralization of DOC and the hydroxyl radical formation is catalyzed by the presence of NO3− (Haag and Hoigne 1985; Molot et al. 2005). However, we found only a weak positive correlation between PD-Ew and NO3 + NO2 (Fig. 4), which was likely due to the high CDOM content in lakes and forest headwater streams (Fig. S2) that interfered with light absorption by nitrate. The CDOM has been shown to absorb irradiation at the same wavelengths as those which induce hydroxyl radical production via NO3−, hence higher concentrations of CDOM suppress NO3− production of hydroxyl radicals (Haag and Hoigne 1985). Likewise, Fe has been shown to increase the water color and absorbance of UV irradiation (Canfield et al. 1984; Maloney et al. 2005), but we found a weak negative correlation between Fe and PD-Ew (Fig. 4). Porcal et al. (2014) found very high photo-mineralization of DOC when the concentration of Fe was high combined with low pH. In our study, the high pH values of river mouths (Fig. S2) could have affected the influence of Fe on PD-Ew because decreases in the hydroxyl radicals (byproduct of Fe photoreaction) have been shown with increases in pH (Zepp et al. 1992; Molot et al. 2005). Overall, these results suggest that pH may be the dominant extrinsic driver that controls the photo-reactivity of DOC along the aquatic continuum in our case, but additional experiments are needed to confirm that this relationship is causal.
When samples were incubated in the laboratory for 365 days, we observed losses in CDOM but no significant change in the PD-Ew between the start and end of the incubation. This shows that when extrinsic factors are held constant, microbial degradation of DOC does not lead to an overall decrease in PD-Ew. However, the microbial processing clearly affected DOC composition, as SUVA254 increased during the microbial incubations. This increase in SUVA254 indicates a net preferential microbial consumption of relatively less colored DOC or production of humic substances by bacteria (Guillemette and del Giorgio 2012). However, in the field data along the land-sea continuum, SUVA254 showed no corresponding increase during transit in the aquatic network, likely because the potential microbial positive effect of SUVA254 was counter-acted by the negative effect on SUVA254 by the photo-processing that occurred in situ (Fasching and Battin 2012). Interestingly, the FRESH index increased significantly both in the natural downstream gradient toward the river mouth and over time in our incubation study, possibly because of bacterial production of fresh fluorescent DOC (Guillemette and del Giorgio 2012).
Thus, in both the in situ and long term incubation measurements we observed significant decreases in CDOM but no continuous significant decrease in PD-Ew over time or along the flow paths. However, in ambient conditions we observed first a significant decrease in PD-Ew, but only from headwater streams to lakes (Fig. 3), after which the PD-Ew increased again toward the river mouths. River mouths showed higher pH than other aquatic ecosystems (Fig. S2), which most likely increased the absorption of light by high molecular weight colored DOC (Pace et al. 2011; Reche et al. 1999). Overall, the most colored DOC fractions were effectively removed from the aquatic ecosystems, reflected for example by the decrease in CDOM along the aquatic continuum. However, as these highly colored DOC molecules were lost photo-reactivity did not decrease but instead showed positive correlations to increasing a254/a365 and FRESH along the land-sea continuum (Fig. 4). This indicates that both low and high molecular weight DOC are photo-reactive, but the quantity of the carbon that undergoes photo-mineralization is influenced by extrinsic variables like pH.
Our study demonstrates how pH may control patterns in the capacity of terrestrial DOC to be photo-mineralized along the freshwater continuum, regardless of the color of the DOC. This challenges the view that DOC photo-reactivity in nature is tightly linked to its ability to absorb light, and suggests that the photo-reactive DOC pool is susceptible to changes in pH along the aquatic continuum. These findings imply that (1) a substantial fraction of the carbon flowing in inland waters remains highly degradable throughout the continental freshwaters network and (2) that rivers act as a potential contributor of highly photo-reactive DOC to receiving coastal and marine systems. This study improves our understanding of the role of freshwaters in regional carbon budgets under scenarios of changing delivery of carbon from land to water.
Change history
26 February 2020
The original publication of this paper contains a mistake in Figure��3.
References
Ågren A, Buffam I, Berggren M, Bishop K, Jansson M, Laudon H (2008) Dissolved organic carbon characteristics in boreal streams in a forest-wetland gradient during the transition between winter and summer. J Geophys Res Biogeosci 113:G03031
Anesio AM, Granéli W (2003) Increased photoreactivity of DOC by acidification: implications for the carbon cycle in humic lakes. Limnol Oceanogr 48:735–744
Benner R, Kaiser K (2011) Biological and photochemical transformations of amino acids and lignin phenols in riverine dissolved organic matter. Biogeochemistry 102:209–222
Bertilsson S, Tranvik LJ (2000) Photochemical transformation of dissolved organic matter in lakes. Limnol Oceanogr 45:753–762
Canfield DE, Linda SB, Hodgson LM (1984) Relations between color and some limnological characteristics of Florida lakes. JAWRA J Am Water Resour Assoc 20:323–329
Cole JJ, Prairie YT, Caraco NF, McDowell WH, Tranvik LJ, Striegl RG, Duarte CM, Kortelainen P, Downing JA, Middelburg JJ, Melack J (2007) Plumbing the global carbon cycle: integrating inland waters into the terrestrial carbon budget. Ecosystems 10:171–184
Cory RM, McNeill K, Cotner JP, Amado A, Purcell JM, Marshall AG (2010) Singlet oxygen in the coupled photochemical and biochemical oxidation of dissolved organic matter. Environ Sci Technol 44:3683–3689
Cory RM, Crump BC, Dobkowski JA, Kling GW (2013) Surface exposure to sunlight stimulates CO2 release from permafrost soil carbon in the Arctic. Proc Natl Acad Sci USA 110:3429–3434
Cory RM, Ward CP, Crump BC, Kling GW (2014) Sunlight controls water column processing of carbon in arctic fresh waters. Science 345:925–928
Creed IF, McKnight DM, Pellerin BA, Green MB, Bergamaschi BA, Aiken GR, Burns DA, Findlay SEG, Shanley JB, Striegl RG, Aulenbach BT, Clow DW, Laudon H, McGlynn BL, McGuire KJ, Smith RA, Stackpoole SM (2015) The river as a chemostat: fresh perspectives on dissolved organic matter flowing down the river continuum. Can J Fish Aquat Sci 72:1272–1285
Cuthbert ID, del Giorgio P (1992) Toward a standard method of measuring color in freshwater. Limnol Oceanogr 37:1319–1326
Fasching C, Battin TJ (2012) Exposure of dissolved organic matter to UV-radiation increases bacterial growth efficiency in a clear-water Alpine stream and its adjacent groundwater. Aquat Sci 74:143–153
Frey KE, McClelland JW (2009) Impacts of permafrost degradation on arctic river biogeochemistry. Hydrol Process 23:169–182
Frey KE, Smith LC (2005) Amplified carbon release from vast West Siberian peatlands by 2100. Geophys Res Lett 32: L09401
Gennings C, Molot LA, Dillon PJ (2001) Enhanced photochemical loss of organic carbon in acidic waters. Biogeochemistry 52:339–354
Gonsior M, Schmitt-Kopplin P, Bastviken D (2013) Depth-dependent molecular composition and photo-reactivity of dissolved organic matter in a boreal lake under winter and summer conditions. Biogeosciences 10:6945–6956
Gonsior M, Hertkorn N, Conte MH, Cooper WJ, Bastviken D, Druffel E, Schmitt-Kopplin P (2014) Photochemical production of polyols arising from significant photo-transformation of dissolved organic matter in the oligotrophic surface ocean. Mar Chem 163:10–18
Granéli W, Lindell M, Tranvik L (1996) Photo-oxidative production of dissolved inorganic carbon in lakes of different humic content. Limnol Oceanogr 41:698–706
Gu Y, Lensu A, Perämäki S, Ojala A, Vähätalo AV (2017) Iron and pH regulating the photochemical mineralization of dissolved organic carbon. ACS Omega 2:1905–1914
Guillemette F, del Giorgio PA (2012) Simultaneous consumption and production of fluorescent dissolved organic matter by lake bacterioplankton. Environ Microbiol 14:1432–1443
Haag WR, Hoigne J (1985) Photo-sensitized oxidation in natural-water via. OH radicals. Chemosphere 14:1659–1671
Hu CM, Muller-Karger FE, Zepp RG (2002) Absorbance, absorption coefficient, and apparent quantum yield: a comment on common ambiguity in the use of these optical concepts. Limnol Oceanogr 47:1261–1267
IPCC (2013) Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York
Koehler B, Landelius T, Weyhenmeyer GA, Machida N, Tranvik LJ (2014) Sunlight-induced carbon dioxide emissions from inland waters. Glob Biogeochem Cycles 28:696–711
Koehler B, Broman E, Tranvik LJ (2016) Apparent quantum yield of photochemical dissolved organic carbon mineralization in lakes. Limnol Oceanogr 61:2207–2221
Lapierre JF, del Giorgio PA (2014) Partial coupling and differential regulation of biologically and photochemically labile dissolved organic carbon across boreal aquatic networks. Biogeosciences 11:5969–5985
Larson RA, Weber EJ (1994) Reaction mechanisms in environmental organic chemistry. Lewis Publishers, Boca Raton
Maloney KO, Morris DP, Moses CO, Osburn CL (2005) The role of iron and dissolved organic carbon in the absorption of ultraviolet radiation in humic lake water. Biogeochemistry 75:393–407
McKnight DM, Boyer EW, Westerhoff PK, Doran PT, Kulbe T, Andersen DT (2001) Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol Oceanogr 46:38–48
Molot LA, Dillon PJ (1997) Photolytic regulation of dissolved organic carbon in northern lakes. Glob Biogeochem Cycles 11:357–365
Molot LA, Hudson JJ, Dillon PJ, Miller SA (2005) Effect of pH on photo-oxidation of dissolved organic carbon by hydroxyl radicals in a coloured, softwater stream. Aquat Sci 67:189–195
Mostofa KMG, Liu C-Q, Vione D, Mottaleb MA, Ogawa H, Tareq SM, Yoshioka T (2013) Colored and chromophoric dissolved organic matter in natural waters. In: Mostofa MGK, Yoshioka T, Mottaleb A, Vione D (eds) Photobiogeochemistry of organic matter: principles and practices in water environments. Springer, Berlin
Murphy KR, Butler KD, Spencer RG, Stedmon CA, Boehme JR, Aiken GR (2010) Measurement of dissolved organic matter fluorescence in aquatic environments: an interlaboratory comparison. Environ Sci Technol 44:9405–9412
Pace ML, Reche I, Cole JJ, Fernández-Barbero A, Mazuecos IP, Prairie YT (2011) pH change induces shifts in the size and light absorption of dissolved organic matter. Biogeochemistry 108:109–118
Panneer Selvam B, Natchimuthu S, Arunachalam L, Bastviken D (2014) Methane and carbon dioxide emissions from inland waters in India—implications for large scale greenhouse gas balances. Glob Chang Biol 20:3397–3407
Panneer Selvam B, Laudon H, Guillemette F, Berggren M (2016) Influence of soil frost on the character and degradability of dissolved organic carbon in boreal forest soils. J Geophys Res Biogeosci 121:829–840
Parlanti E, Worz K, Geoffroy L, Lamotte M (2000) Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org Geochem 31:1765–1781
Porcal P, Dillon PJ, Molot LA (2014) Interaction of extrinsic chemical factors affecting photodegradation of dissolved organic matter in aquatic ecosystems. Photochem Photobiol Sci 13:799–812
Reche I, Pace ML, Cole JJ (1999) Relationship of trophic and chemical conditions to photobleaching of dissolved organic matter in lake ecosystems. Biogeochemistry 44:259–280
Tranvik LJ, Downing JA, Cotner JB, Loiselle SA, Striegl RG, Ballatore TJ, Dillon P, Finlay K, Fortino K, Knoll LB, Kortelainen PL, Kutser T, Larsen S, Laurion I, Leech DM, McCallister SL, McKnight DM, Melack JM, Overholt E, Porter JA, Prairie Y, Renwick WH, Roland F, Sherman BS, Schindler DW, Sobek S, Tremblay A, Vanni MJ, Verschoor AM, von Wachenfeldt E, Weyhenmeyer GA (2009) Lakes and reservoirs as regulators of carbon cycling and climate. Limnol Oceanogr 54:2298–2314
Vachon D, Lapierre JF, del Giorgio PA (2016) Seasonality of photochemical dissolved organic carbon mineralization and its relative contribution to pelagic CO2 production in northern lakes. J Geophys Res Biogeosci 121:864–878
Vahatalo AV, Salkinoja-Salonen M, Taalas P, Salonen K (2000) Spectrum of the quantum yield for photochemical mineralization of dissolved organic carbon in a humic lake. Limnol Oceanogr 45:664–676
Weishaar JL, Aiken GR, Bergamaschi BA, Fram MS, Fujii R, Mopper K (2003) Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ Sci Technol 37:4702–4708
Weyhenmeyer GA, Fröberg M, Karltun E, Khalili M, Kothawala D, Temnerud J, Tranvik LJ (2012) Selective decay of terrestrial organic carbon during transport from land to sea. Glob Change Biol 18:349–355
Zafiriou OC, Joussotdubien J, Zepp RG, Zika RG (1984) Photochemistry of natural-waters. Environ Sci Technol 18:A358–A371
Zepp RG, Hoigne J, Bader H (1987) Nitrate-induced photooxidation of trace organic chemicals in water. Environ Sci Technol 21:443–450
Zepp RG, Faust BC, Hoigne J (1992) Hydroxyl radical formation in aqueous reactions (pH 3–8) of iron(II) with hydrogen peroxide: the photo-Fenton reaction. Environ Sci Technol 26:313–319
Ziegler S, Benner R (2000) Effects of solar radiation on dissolved organic matter cycling in a subtropical seagrass meadow. Limnol Oceanogr 45:257–266
Zsolnay A, Baigar E, Jimenez M, Steinweg B, Saccomandi F (1999) Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 38:45–50
Acknowledgements
Open access funding provided by Lund University. We would like to thank Lina Allesson, Marcin Jackowicz-Korczynski, Mårten Dario and Julia Jakobsson for their assistance in sample analysis. Sivakiruthika Natchimuthu, Ida Taberman, Peder Blomkvist, Marcus Klaus, Anders Jonsson and Abisko scientific research station for their assistance in sample collection. The data is available by requesting the corresponding author. The study was financed by Helge Ax:son Johnsons Stiftelse (No. 130622) and Kungliga Fysiografiska Sällskapet i Lund (No. 32953) Grants to B.P.S. and by a FORMAS (No. 239-2014-698) Grant to M.B. Finally, J.K. was supported by FORMAS (No. 210-2012-1461) and D.B. by Swedish Research Council VR (No. 2012-00048).
Author information
Authors and Affiliations
Contributions
BPS and MB designed the study; BPS, ARAS, DB and JK conducted field work; BPS conducted lab work and analyzed the data; BPS wrote the first draft of the manuscript with major contributions from MB and J-FL; JK, DB and ARAS commented and edited several revised versions of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Panneer Selvam, B., Lapierre, JF., Soares, A.R.A. et al. Photo-reactivity of dissolved organic carbon in the freshwater continuum. Aquat Sci 81, 57 (2019). https://doi.org/10.1007/s00027-019-0653-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-019-0653-0