Abstract
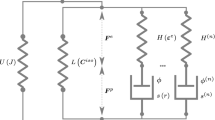
In this paper, we develop a thermodynamic framework that is capable of describing the response of viscoelastic materials that are undergoing chemical reactions that takes into account stoichiometry. Of course, as a special sub-case, we can also describe the response of elastic materials that undergo chemical reactions. The study generalizes the framework developed by Rajagopal and co-workers to study the response of a disparate class of bodies undergoing entropy producing processes. One of the quintessential feature of this framework is that the second law of thermodynamics is formulated by introducing Gibbs’ potential, which is the natural way to study problems involving chemical reactions. The Gibbs potential–based formulation also naturally leads to implicit constitutive equations for the stress tensor. Another feature of the framework is that the constraints due to stoichiometry can also be taken into account in a consistent manner. The assumption of maximization of the rate of entropy production due to dissipation, heat conduction, and chemical reactions is invoked to determine an equation for the evolution of the natural configuration κ p(t)(B), the heat flux vector and a novel set of equations for the evolution of the concentration of the chemical constituents. To determine the efficacy of the framework with regard to chemical reactions, those occurring during vulcanization, a challenging set of chemical reactions, are chosen. More than one type of reaction mechanism is considered and the theoretically predicted distribution of mono, di and polysulfidic cross-links agree reasonably well with available experimental data.
Similar content being viewed by others
References
Abhilash P.M., Kannan K., Varkey B.: Simulation of curing of a slab of rubber. Mater. Sci. Eng. B 168, 237–241 (2010)
Bjornbom P.H.: The independent reactions in calculations of complex chemical equilibria. Ind. Eng. Chem. Fundam. 14, 102–106 (1975)
Brinkley S.R.: Note on conditions of equilibrium for systems of many constituents. J. Chem. Phys. 14, 563–564 (1946)
Chong E.K.P., Zak S.H.: An Introduction to Optimization. Wiley (Asia) Pte. Ltd., Singapore (2004)
Coran A.Y.: Vulcanization. part vi. a model and treatment for scorch delay kinetics. Rubber Chem. Technol. 37, 689–697 (1964)
Coran, A.Y.: Vulcanization. In: Mark, J.E., Erman, B., Eirich, F.R. (eds.) The Science and Technology of Rubber, pp. 321–367. (2005)
Denbigh K.: The Principles of Chemical Equilibrium. Cambridge University Press, London (1971)
Ding R., Leonov A.: A kinetic model for the sulfur accelerated vulcanization of rubber compound. J. Appl. Polym. Sci. 69, 455–463 (1996)
Ding R., Leonov A., Coran A.Y.: A study of the vulcanization kinetics of an accelerated-sulfur sbr compound. Rubber Chem. Technol. 69, 81–91 (1996)
Eckart C.: The thermodynamics of irreversible processes. IV. The theory of elasticity and anelasticity. Phys. Rev. 73, 373–382 (1948)
Fan R., Zhang Y., Huang C., Gong P., Zhang Y.: Simulation and verification for sulfur accelerated vulcanization of gum natural rubber compound. Rubber Chem. Technol. 75, 287–297 (2002)
Geyser M., McGill W.J.: A study of the rate of formation of polysulfides of tetramethylthiuram disulfide. J. Appl. Polym. Sci. 55, 215–224 (1995)
Geyser M., McGill W.J.: Thiuram-accelerated sulfur vulcanization. I. The formation of the active sulfurating agent. J. Appl. Polym. Sci. 60, 425–430 (1996)
Geyser M., McGill W.J.: Thiuram-accelerated sulfur vulcanization. II. The formation of crosslink precursors. J. Appl. Polym. Sci. 60, 431–437 (1996)
Ghosh P., Katare S., Patkar P., Caruthers J.M., Venkatasubramanian V., Walker K.A.: Sulphur vulcanization of natural rubber for benzothiazole accelerated formulations: From reaction mechanisms to a rational kinetic model. Rubber Chem. Technol. 76, 592–693 (2003)
Green A.E., Nagdhi P.M.: On the thermodynamics and the nature of the second law. Proc. R. Soc. A 357, 253–270 (1977)
Jog C.S.: Continuum Mechanics. Narosa Publishing House Pvt. Ltd., New Delhi (2007)
Kannan K., Rajagopal K.R.: A thermomechanical framework for the transition of a viscoelastic liquid to a viscoelastic solid. Math. Mech. Solids 9, 37–59 (2004)
Loo C.T.: High temperature vulcanization of elastomers: 2. Network structures in conventional sulphenamide-sulphur natural rubber vulcanizates. Polymer 15, 357–365 (1974)
Loo C.T.: High temperature vulcanization of elastomers: 3. Network structures of efficiently vulcanized natural rubber mixes. Polymer 15, 729–737 (1974)
Malek J., Rajagopal K.R.: A thermodynamic framework for a mixture of two liquids. Nonlinear Anal. Real World Appl. 9, 1649–1660 (2008)
Malik, W.A., Rajagopal, S., Darbha, S., Rajagopal, K.R. Maximizing the algebraic connectivity with at most a prescribed number of edges. In: Sivasundaram, S., Vasundhara, J., Udwadia F.E., Lasiecka, I. (eds.) Advances in Dynamics and Controls: Theory, Methods and Applications. (2009)
Moakher M.: Fourth-order cartesian tensors: Old and new facts, notions and applications. Q. J. Mech. Appl. Math. 61, 181–203 (2008)
Morgan B., McGill W.J.: Benzothiazole accelerated sulfur vulcanization. iv. effect of zno and bis(2 mercaptobenzothiazole)zinc(ii) on 2 bisbenzothiazole 2,2 polysulfide formation in 2 bisbenzothiazole 2,2 disulfide and 2 bisbenzothiazole 2,2 disulfide/sulfur. J. Appl. Polym. Sci. 76, 1405–1412 (2000)
Morgan B., McGill W.J.: Benzothiazole accelerated sulfur vulcanization. v. 2 bisbenzothiazole 2,2 disulfide/zno and 2 bisbenzothiazole 2,2 disulfide/(2 mercaptobenzothiazole)zinc(ii) as accelerators for 2,3 dimethyl 2 butene. J. Appl. Polym. Sci. 76, 1413–1421 (2000)
Onsager L.: Reciprocal relations in irreversible thermodynamics. Phys. Rev. 37, 405–426 (1931)
Prasad S.C., Rajagopal K.R.: A continuum model for the creep of single crystal nickel-base superalloys. Acta Mater. 53, 669–679 (2005)
Rajagopal K.R., Srinivasa A.R.: A thermodynamic frame work for rate type fluid models. J. Nonnewton. Fluid Mech. 88, 207–227 (2000)
Rajagopal K.R., Srinivasa A.R.: On the thermomechanics of materials that have multiple natural configurations - part i: Viscoelasticity and classical plasticity. Zeitschrift für Angew. Mathematik Phys. 55, 861–893 (2004)
Rajagopal K.R., Srinivasa A.R.: On the thermomechanics of materials that have multiple natural configurations- part ii: Twinning and solid to solid phase transformation. Zeitschrift für Angew. Mathematik Phys. 55, 1074–1093 (2004)
Rajagopal K.R., Srinivasa, A.R.: A gibbs-potential-based formulation for obtaining the response functions for a class of viscoelastic materials. Proceedings of the Royal Society A, pp. 1–20. (2010)
Rajagopal K.R., Tao L.: Mechanics of Mixtures. World Scientific Publishing Co. Pte Ltd., Singapore (1995)
Rajagopal K.R., Tao L.: Modeling of the microwave drying process of aqueous dielectrics.. Zeitschrift für Angew. Mathematik Phys. 53, 923–948 (2002)
Rao I.J., Rajagopal K.R.: A thermodynamic framework for the study of crystallization in polymers. Zeitschrift für Angew. Mathematik Phys. 53, 365–406 (2002)
Schrodinger E.: What is Life?. Cambridge University Press, Cambridge (1992)
Smith G.F.: On isotropic integrity basis. Arch. Ration. Mech. Anal. 18, 282–292 (1965)
Spencer A.J.M., Rivlin R.S.: The theory of matrix polynomials and its application to the mechanics of isotropic continua. Arch. Ration. Mech. Anal. 2, 309–336 (1958)
Tao, L., Rajagopal, K.R.: On the construction of constitutive relations in hyperelasticity. In preparation
Truesdell C., Noll W.: The Non-linear Field Theories of Mechanics. Springer, Berlin (1992)
Zeleznik F.J., Gordon S.: Calculations of complex chemical equilibria. Ind. Eng. Chem. 60, 27–57 (1968)
Ziegler, H.: Some extremum principles in irreversible thermodynamics with application to continuum mechanics. In: Sneddon I.N., Hill R. (eds.) Progress in Solid Mechanics, vol. 4, pp. 3–113. (1963)
Ziegler H., Wehrli C.: The derivation of constitutive relations from the free-energy and the dissipation function. Adv. Appl. Mech. 25, 183–238 (1987)
Author information
Authors and Affiliations
Corresponding author
Additional information
Submitted to Zeitschrift für Angewandte Mathematik und Physik (ZAMP).
Rights and permissions
About this article
Cite this article
Kannan, K., Rajagopal, K.R. A thermodynamical framework for chemically reacting systems. Z. Angew. Math. Phys. 62, 331–363 (2011). https://doi.org/10.1007/s00033-010-0104-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00033-010-0104-1