Abstract
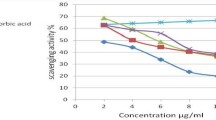
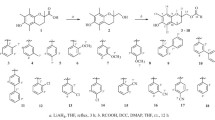
Synthesis, antioxidant activity, and quantitative structure–activity relationship (QSAR) of 25 of chalcone derivatives is reported here. They were synthesized by Claisen–Schmidt reaction and were characterized by FTIR, NMR, and mass spectroscopy. Antioxidant activity is evaluated through four different methods namely, superoxide radical-scavenging, hydrogen peroxide scavenging, reducing power, and DPPH radical-scavenging assays. Generally, compounds with –SCH3 and –OCH3 in the para position of the A-ring and –OH in the B-ring were more active than others. In few cases some of the compounds were more active than ascorbic acid or butylated hydroxytoluene. QSAR was developed correlating the antioxidant activity with the structural features of the compounds and the predictive capability of the models was estimated using internal and external validation methods. All the predictions were within the 99% confidence level. Spatial, structural, and lipophilic properties of the compounds determine their antioxidant properties.







Similar content being viewed by others
Notes
Selected spectral data for C24: IR (KBr) 1652 cm−1(Carbonyl), 1H NMR (400 MHz, CDCl3): δ 3.84 (s, 3H), δ 6.90–6.92 (m, 2H), δ 7.14 (ddd, 1H, J = 8.0 Hz, J′ = 2.4 Hz, J″ = 0.8 Hz), δ 7.36 (dd, 1H, J = 8.4 Hz, J′ = 7.6 Hz), δ 7.38 (d, 1H, J = 15.6 Hz), δ 7.54–7.58 (m, 3H), δ 7.66 (dd, 1H, J = 2.4 Hz, J′ = 1.6 Hz), δ 7.79 (d, 1H, J = 16 Hz).13C NMR (100 MHz, CDCl3): δ 191.00, 161.84, 156.56, 145.45, 139.73, 130.41, 129.83, 127.47, 120.79, 120.33, 119.58, 115.25, 114.45, 55.424. EIMS: [M]+ 254.37.
References
Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 90:7915–7922
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bednarczyk D, Ekins S, Wikel JH, Wright SH (2003) Influence of molecular structure on substrate binding to the human organic cation transporter, hOCT1. Mol Pharmacol 63:489–498
Caballero J, Zampini FM, Collina S, Fernandez M (2007) Quantitative structure–activity relationship modeling of growth hormone secretagogues agonist activity of some tetrahydroisoquinoline 1-carboxamides. Chem Biol Drug Des 69:48–55
Cartling B, Ehrenberg A (1978) A molecular mechanism of the energetic coupling of a sequence of electron transfer reactions to endergonic reactions. Biophys J 23:451–461
Cioffi G, Escobar LM, Braca A, Tommasi ND (2003) Antioxidant chalcone glycosides and flavanones from Maclura(Chlorophora)tinctoria. J Nat Prod 66:1061–1064
Dhar DN (1981) The chemistry of chalcones and related compounds, vol 213. Wiley, New York
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Durand AC, Farce A, Carato P, Dilly S, Yous S, Berthelot P, Chavatte P (2007) Quantitative structure–activity relationships studies of antioxidant hexahydropyridoindoles and flavonoid derivatives. J Enzyme Inhib Med Chem 22:556–562
Epsin JC, Soler-Rivas C, Wichers HJ (2000) Anthocyanin-based natural colorants: a new source of antiradical activity for foodstuff. J Agric Food Chem 48:648–656
Farkas O, Jakus J, Heberger K (2004) Quantitative structure–antioxidant activity relationships of flavonoid compounds. Molecules 9:1079–1088
Flamm ES, Demopoulos HB, Seligman ML, Poser RG, Ransohoff J (1978) Free radicals in cerebral ischemia. Stroke 9:445–447
Foti MC (2007) Antioxidant properties of phenols. J Pharm Pharmacol 59:1673–1685
Fujii J, Taniguchi N (1999) Down regulation of superoxide dismutases and glutathione peroxidase by reactive oxygen and nitrogen species. Free Radic Res 31:301–308
Green AR, Shuaib A (2006) Therapeutic strategies for the treatment of stroke. Drug Discov Today 11:681–693
Gutteridge JM (1993) Free radicals in disease processes: a compilation of cause and consequence. Free Radic Res Commun 19:141–158
Hobza P, Kabelac M, Sponer J, Mejzlik P, Vondrasek J (1997) Performance of empirical potentials (AMBER, CFF95, CVFF, CHARMM, OPLS, POLTEV), semiempirical quantum chemical methods (AM1, MNDO/M, PM3) and ab initio Hartree-Fock method for interaction of DNA bases. Comparison with nonempirical beyond-Hartree-Fock results. J Comput Chem 18:1136–1150
Hu CC, Lin JT, Lu FJ, Chou FP, Yang DJ (2007) Determination of carotenoids in Dunaliella salina cultivated in Taiwan and antioxidant capacity of the algal carotenoid extract. Food Chem 109:439–446
Karelson M (2000) Molecular Descriptors in QSAR/QSPR. Wiley-Interscience, New York
Kiemele MJ, Schmidt SR, Berdine RJ (1997) Basic statistics tools for continuous improvement, vol 7, 4th edn. Air academy press, Colorado springs, Colorado, p 3
Kozlowski D, Trouillas P, Calliste C, Marsal P, Lazzaroni R, Duroux JL (2007) Density functional theory study of the conformational, electronic, and antioxidant properties of natural chalcones. J Phys Chem A 111:1138–1145
Lamson DW, Brignall MS (1999) Antioxidants in cancer therapy; their actions and interactions with oncologic therapies. Altern Med Rev 4:304–329
Lin J, Rivett DE, Wilshire JFK (1977) The preparation and photochemical properties of some 1,3-diphenyl-2-pyrazolines containing a heteroaromatic substituent. Aust J Chem 30:629–637
Martinez AC, Marcelo EL, Marco AO, Moacyr M (2001) Differential responses of superoxide dismutase in freezing resistant Solanum curtibolam and freezing sensitive Solanum tuberosum subjected to oxidative and water stress. Plant Sci 160:505–515
Mattson MP (2003) Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromol Med 3:65–94
Olinski R, Gackowski D, Foksinski M, Rozalski R, Roszkowski K, Jaruga P (2002) Oxidative DNA damage: assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radic Biol Med 33:192–200
Ou B, Huang D, Woodill MH, Flanagan JA, Deemer EK (2002) Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: a comparative study. J Agric Food Chem 50:3122–3128
Oyaizu M (1986) Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:307–315
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63:1035–1043
Rice-Evans CA, Miller NJ, Paganga G (1997) Structure–antioxidant activity relationships of flavonoids and phenolic acids. Trends Plant Sci 2:152–159
Rogers D, Hopfinger AJ (1994) Application of genetic function approximation to quantitative structure–activity relationships and quantitative structure–property relationships. J Chem Inf Comput Sci 34:854–866
Rohrbaugh RH, Jurs PC (1987) Molecular shape and the prediction of high-performance liquid chromatographic retention indexes of polycyclic aromatic hydrocarbons. Anal Chim Acta 199:99–109
Ruch JR, Crist KA, Klaunig JE (1989) Effects of culture duration on hydrogen peroxide-induced hepatocyte toxicity. Toxicol Appl Pharmacol l00:45l–464
Satyanarayana K, Rao MNA (1993) Antiinflamatory, analgesic, and antipyretic activities of 3-[4-[3-(4-dimethylaminophenyl)-1-oxo]-2-propenyl]sydnone. Indian Drugs 30:313–318
Shen L, Ji HF, Zhang HY (2007) How to understand the dichotomy of antioxidants. Biochem Biophys Res Commun 362:543–545
Shibata S (1994) Anti-tumorigenic chalcones. Stem Cells 12:44–52
Sivakumar PM, Geetha Babu SK, Doble M (2007a) QSAR studies on chalcones and flavonoids as anti-tuberculosis agents using genetic function approximation (GFA) method. Chem Pharm Bull 55:44–50
Sivakumar PM, Seenivasan SP, Kumar V, Doble M (2007b) Synthesis, antimycobacterial activity evaluation, and QSAR studies of chalcone derivatives. Bioorg Med Chem Lett 17:1695–1700
Stanton DT, Jurs PC (1990) Development and use of charged partial surface area structural descriptors in computer-assisted quantitative structure–property relationship studies. Anal Chem 62:2323–2329
Stavrovskaya IG, Kristal BS (2005) The powerhouse takes control of the cell: is the mitochondrial permeability transition a viable therapeutic target against neuronal dysfunction and death? Free Radic Biol Med 38:687–697
Todeschini R, Consonni V (2000) Handbook of molecular descriptors. Wiley-VCH, Weinheim
Todeschini R, Lasagni M, Marengo E (1994) New molecular descriptors for 2D- and 3D-structures. J Chemom 8:263–273
Varma SD, Devamanoharan PS, Morris SM (1995) Prevention of cataracts by nutritional and metabolic antioxidants. Crit Rev Food Sci Nutr 35:111–129
Vaya J, Belinky PA, Aviram M (1997) Antioxidant constituents from licorice roots: isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radic Biol Med 23:302–313
Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ (2003) Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 361:2017–2023
Witte P, Beuerle F, Hartnagel U, Lebovitz R, Savouchkina A, Sali S, Guldi D, Chronakis N, Hirsch A (2007) Water solubility, antioxidant activity and cytochrome C binding of four families of exohedral adducts of C60 and C70. Org Biomol Chem 5:3599–3613
Yamagami C, Akamatsu M, Motohashi N, Hamada S, Tanahashi T (2005) Quantitative structure activity relationship studies for antioxidanthydroxybenzalacetones by quantum chemicaland3-D-QSAR (CoMFA) analyses. Bioorg Med Chem Lett 15:2845–2850
Yayli N, Ucuncu O, Aydin E, Gok Y, Yasar A, Baltaci C, Yildirim N, Kucuk M (2005) Intramolecular 4π photocyclization of chalconoid-like compounds in solution and antimicrobial activities. J Photochem Photobiol A 169:229–234
Yayli N, Ucuncu O, Yasar A, Kucuk M, Yayli N, Akyuz E, Karaoglu SA (2006) Synthesis and biological activities of N-alkyl derivatives of o-, m-, and p-nitro (E)-4-azachalcones and stereoselective photochemistry in solution, with theoretical calculations. Turk J Chem 30:505–514
Yu L, Haley S, Perret J, Harris M, Wilson J, Qian M (2002) Free radical scavenging properties of wheat extracts. J Agric Food Chem 50:1619–1624
Ziakas GN, Rekka EA, Gavalas AM, Eleftheriou PT, Kourounakis PN (2006) New analogues of butylated hydroxytoluene as anti-inflammatory and antioxidant agents. Bioorg Med Chem 14:5616–5624
Acknowledgment
Authors thank SAIF, IIT Madras for providing spectral analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sivakumar, P.M., Prabhakar, P.K. & Doble, M. Synthesis, antioxidant evaluation, and quantitative structure–activity relationship studies of chalcones. Med Chem Res 20, 482–492 (2011). https://doi.org/10.1007/s00044-010-9342-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9342-1