Abstract
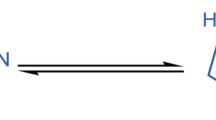
A series of 6,7,8-substituted thiosemicarbazones (2a–j) of 2-chloro-3-formyl-quinoline derivatives were cyclized to the title compounds (3a–j) using acetic anhydride. The structures of the final compounds (3a–j) were confirmed by elemental and spectral (IR, 1H NMR and MS) analysis. Some of the title compounds have shown promising anticancer and antitubercular activities.




Similar content being viewed by others
References
Doyle A, Griffiths JB (2000) Cell and tissue culture for medical research. Wiley, Academic Press, London
Hirao K, Shinohara Yoshitaka, Hiroyuki T, Fukushima S, Takahashi M, Ito N (1976) Carcinogenic activity of quinoline on rat liver. Cancer Res 36:329–335
Kategaonkar A, Shinde PV, Kategaonkar AH, Pasale SK, Shingate BB (2010) Synthesis and biological evaluation of new 2-chloro-3-((4-phenyl-1H-1, 2, 3-triazol-yl)-methyl)-quinoline derivatives via click chemistry approach. Eur J Med Chem 45:3142–3146
Lamani RS, Shetty NS, Kamble RR, Khazi IA (2009) Synthesis and antimicrobial studies of novel methylene bridged benzisoxazolyl imidazo-[2,1-b][1,3,4]-thiadiazole derivatives. Eur J Med Chem 44:2828–2833
Leclerc G, Marciniak G, Decker N, Schwartz J (1986) Cardiotonic agents. 1. Synthesis and structure activity relationships in a new class of 3-, 4-, and 5-pyridyl-2(1H)-quinolone derivatives. J Med Chem 29:2427–2432
Lilienkampf A, Mao J, Wan B, Wang Y, Franzblau SG, Kozikowski AP (2009) Structure–activity relationships for a series of quinoline-based compounds active against replicating and nonreplicating Mycobacterium tuberculosis. J Med Chem 52:2109–2118
Lipinski A, Franco L, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23:3–25
Meng XW, Lei F, Xue QL, Xue-Zhang Z, Zhi HS (2009) Synthesis of new chiral 2,5-disubstituted 1,3,4-thiadiazoles possessing γ-butenolide moiety and preliminary evaluation of in vitro anticancer activity. Eur J Med Chem 44:3340–3344
Mital A, Negi VS, Ramachandran U (2006) Synthesis and antimycobacterial activities of certain trifluoromethyl-aminoquinoline derivatives. ARKIVOC x:220–227
Nayar A, Jain R (2008) Synthesis and anti-tuberculosis activity of 2,4-disubstituted quinolines. Indian J Chem 47B:117–128
Otto MC, Bramha NA (1978) New synthesis of quinoline, thienopyrimidine and related fused pyridines. Tetrahedron Lett 23:2045–2048
Otto MC, Bramha NA (1981) A versatile new synthesis and related fused pyridines. Part 5. The synthesis of 2-chloroquinoline-3-carbaldehydes. J Chem Soc Perkin Trans 1:1520–1530
Padmavathi V, Reddy SN, Reddy GD, Padmaja A (2010) Synthesis and bioassay of aminosulfonyl-1,3,4-oxadiazoles and their interconversion to 1,3,4-thiadiazoles. Eur J Med Chem 45(9):4246–4251
Pokalwar RU, Hangarge RV, Maskeb PV, Shingare MS (2006) Synthesis and antibacterial activities of α-hydroxyphosphonates and acetyloxyphosphonates derived from 2-chloroquinoline-3-carbaldehyde. ARKIVOC xii:196–204
Solak S, Rollas S (2006) Synthesis and antituberculosis activity of 2-(aryl/alkylamino)-5-(4-aminophenyl)-1,3,4-thiadiazoles and their Schiff bases. ARKIVOC 12:173–181
Stewart JP (1989) Optimization of parameters for semi empirical methods I. Method J Comput Chem 10:209–220
Stewart JP (1990) MOPAC 6.0 QCPE 455. Indiana University, Bloomington (IN 47405)
Vangapandu S, Jain M, Jain R, Kaur S, Singh PP (2004) Ring-substituted quinolines as potential anti-tuberculosis agents. Bioorg Med Chem 12:2501–2508
Acknowledgments
The authors are thankful to the USIC, Karnatak University, Dharwad for carrying out the spectral (IR, 1H NMR, MS) and elemental analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marganakop, S.B., Kamble, R.R., Taj, T. et al. An efficient one-pot cyclization of quinoline thiosemicarbazones to quinolines derivatized with 1,3,4-thiadiazole as anticancer and anti-tubercular agents. Med Chem Res 21, 185–191 (2012). https://doi.org/10.1007/s00044-010-9522-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9522-z