Abstract
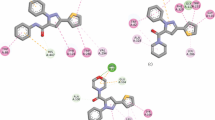
A new series of 1-acetyl-3-aryl thioureas (3f1–15) was synthesized by the reaction of acetyl isothiocyanate with a variety of suitably substituted aromatic anilines. The acetyl isothiocyanate was freshly prepared by reaction of corresonding acid chloride with potassium thiocyanate. The structural confirmation of all compounds was carried out by spectroscopic techniques and in case of 3a by X-ray diffraction study. The newly prepared compounds were subjected to computational studies and evaluated for their cholinesterase (acetylcholinesterase and butyrylcholinesterase) inhibition studies. Except 3f9 and 3f15, all the derivatives were found as selective inhibitor of acetylcholinesterase. Compound 3f2 (IC50 ± SEM = 1.99 ± 0.11 µM) was found to be the most potent inhibitor of acetylcholinesterase exhibited ≈11 times greater inhibitory potential than reference inhibitor i.e. neostigmine (IC50 ± SEM = 22.2 ± 3.2 µM). Compound 3f9 was found to be most potent butyrylcholinesterase inhibitor (IC50 ± SEM = 1.33 ± 0.11 µM), exhibiting ≈four times greater selectivity for butyrylcholinesterase over acetylcholinesterase. Molecular docking studies were carried out to determine the binding site interactions of these potent inhibitors with cholinesterases and also supported the experimental observations.








Similar content being viewed by others
References
Aly AA, Ahmed EK, Mokadem KMEl, Hegazy MEAF (2007) Update survey on aroyl substituted thioureas and their applications. J Sulfur Chem 28:73–93
Brouwer MS, Grosscurt AC, Duphar International Research BV (1982) Urea and thiourea compounds and insecticidal compositions. U.S. Patent 4,350,706
Darvesh S, Hopkins DA, Geula C (2003) Neurobiology of butyrylcholinesterase. Nat Rev Neurosci 4:131–138
Dave KR, Syal AR, Katyare SS (2000) Tissue cholinesterases. A comparative study of their kinetic properties. Z Naturforsch C 55:100–108
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Eweis M, Elkholy S, Elsabee M (2006) Antifungal efficacy of chitosan and its thiourea derivatives upon the growth of some sugar-beet pathogens. Int J Biol Macromol 38:1–8
Fukui K (1982) Chemical reactivity theory-its pragmatism and beyond. Pure Appl Chem 54:1825–1836
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al. (2009) Gaussian. Inc., Wallingford CT
Gökce H, Bahçeli S (2013) FT-IR, Micro-Raman and UV–Vis spectroscopic and quantum chemical investigations of free 2, 2′-dithiodipyridine and its metal (Co, Cu, and Zn) halide complexes. Spectrochim Acta Mol Biomol Spectrosc 114:61–73
Gopi D, Bhuvaneswaran N, Rajeswarai S, Ramadas K (2000) Synergistic effect of thiourea derivatives and non-ionic surfactants on the inhibition of corrosion of carbon steel in acid environments. Anti-Corrosion Method Mater 47:332–339
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Ke SY, Xue SJ (2006) Synthesis and herbicidal activity of N-(o-fluorophenoxyacetyl) thioureas derivatives and related fused heterocyclic compounds. Arkivoc 10:63–68
Koch KR (2001) New chemistry with old ligands: N-alkyl-and N, N-dialkyl-N′-acyl (aroyl) thioureas in co-ordination, analytical and process chemistry of the platinum group metals. Coord Chem Rev 216:473–488
Koelle GB (1994) Pharmacology of organophosphates. J Appl Toxicol 14:105–109
Krieter PA, Ziegler DM, Hill KE, Burk RF (1984) Increased biliary GSSG efflux from rat livers perfused wth thiocarbamide substrates for the flavin-containing monooxygenase. Mol Pharmacol 26:122–127
Larik FA, Saeed A, Channar PA, Ismail H, Dilshad E, Mirza B (2016) New 1-octanoyl-3-aryl thiourea derivatives: solvent-free synthesis, characterization and multi-target biological activities. Bangladesh J Pharmacol 11:894–902
LeadIT B (2011) http://www. biosolveit. de/LeadIT. Accessed 12th Mar
Marrs TC, Maynard R (2013) Neurotranmission systems as targets for toxicants: a review. Cell Biol Toxicol 29:381–396
Mertschenk B, Beck F, Bauer W (2002) Thiourea and thiourea derivatives. Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Berlin
Nachon F, Carletti E, Ronco C, Trovaslet M, Nicolet Y, Jean L, Renard PY (2013) Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer’s drugs targeting acetyl-and butyryl-cholinesterase. Biochem J 453:393–399
Nencki M (1873) Zur kenntniss des sulfoharnstoffs. Eur J Inorg Chem 6:598–600
Ren J, Diprose J, Warren J, Esnouf RM, Bird LE, Ikemizu S, Slater M, Milton J, Balzarini J, Stuart DI (2000) Phenylethylthiazolylthiourea (PETT) non-nucleoside inhibitors of HIV-1 and HIV-2 reverse transcriptases structural and biochemical analyses. J Biol Chem 275:5633–5639
Saeed A, Flörke U, Erben MF (2014) A review on the chemistry, coordination, structure and biological properties of 1-(acyl/aroyl)-3-(substituted) thioureas. J Sulfur Chem 35:318–355
Saeed A, Khera RA, Abbas N, Latif M, Sajid I, Floerke U (2010) Synthesis, characterization, crystal structures, and antibacterial activity of some new 1-(3, 4, 5-trimethoxybenzoyl)-3-aryl thioureasreas. Turk J Chem 34:335–346
Saeed A, Mahesar PA, Zaib S, Khan MS, Matin A, Shahid M, Iqbal J (2014) Synthesis, cytotoxicity and molecular modelling studies of new phenylcinnamide derivatives as potent inhibitors of cholinesterases. Eur J Med Chem 78:43–53
Saeed A, Shaheen U, Hameed A, Naqvi SH (2009) Synthesis, characterization and antimicrobial activity of some new 1-(fluorobenzoyl)-3-(fluorophenyl) thioureas. J Fluor Chem 130(11):1028–1034
Schroeder DC (1955) Thioureas. Chem Rev 55:181–228
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A 64:112–122
Shen Y, Zhang J, Sheng R, Dong X, He Q, Yang B, Hu Y (2009) Synthesis and biological evaluation of novel flavonoid derivatives as dual binding acetylcholinesterase inhibitors. J Enzyme Inhib Med Chem 24:372–380
Smith DA (2009) Treatment of Alzheimer’s disease in the long-term-care setting. AJHP 66(10):899–907
Sriram D, Yogeeswari P, Dinakaran M, Thirumurugan R (2007) Antimycobacterial activity of novel 1-(5-cyclobutyl-1, 3-oxazol-2-yl)-3-(sub) phenyl/pyridylthiourea compounds endowed with high activity toward multidrug-resistant Mycobacterium tuberculosis. J Antimicrob Chemother 59:1194–1196
Studio D, Insight I (2009) Accelrys Software Inc. San Diego, CA, 92121.
Terry AV, Buccafusco JJ (2003) The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharm Exp Ther 306:821–827
Tougu V (2001) Acetylcholinesterase: mechanism of catalysis and inhibition. Curr Med Chem Cent Nerv Syst Agents 1:155–170
Ziegler DM (1978) Intermediate metabolites of thiocarbamides, thioureylenes and thioamides; mechanism of formation and reactivity. Biochem Soc Trans 6:94–96
Ziegler-Skylakakis K, Nill S, Pan JF, Andrae U (1998) S-Oxygenation of thiourea results in the formation of genotoxic products. Environ Mol Mutagen 31:362–373
Acknowledgments
J. Iqbal is thankful to the Organization for the Prohibition of Chemical Weapons (OPCW), The Hague, The Netherlands and Higher Education Commission of Pakistan for the financial support through Project No. 20-3733/NRPU/R&D/14/520 for the financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Saeed, A., Shakil Shah, M., Ali Larik, F. et al. Synthesis, computational studies and biological evaluation of new 1-acetyl-3-aryl thiourea derivatives as potent cholinesterase inhibitors. Med Chem Res 26, 1635–1646 (2017). https://doi.org/10.1007/s00044-017-1829-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1829-6