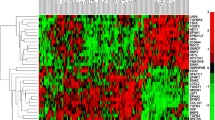
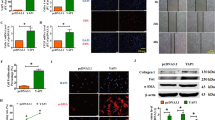
Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and highly lethal fibrotic lung disease with unknown cause or cure. Although some microRNAs (miRNAs), such as miR-26a and let-7d, have been confirmed, the contribution to the pathophysiological processes of IPF, the roles of miRNAs and intrinsic links between them in fibrotic lung diseases are not yet well understood. In this study, we found that Lin28B could induce the process of epithelial-mesenchymal transition (EMT) by inhibiting let-7d, whereas inhibition of Lin28B mitigated TGF-β1-induced fibrogenesis and attenuated EMT in both cultured A549 cells and MLE-12 cells. More importantly, over-expression of miR-26a could simultaneously enhance the expression of let-7d in A549 cells, and further study confirmed that Lin28B was one of the direct targets of miR-26a, which mediates, at least in part, the regulatory effects of miR-26a on the biogenesis of let-7d. Finally, we constructed a regulatory network among miRNAs involved in the progression of IPF. Taken together, our study deciphered the essential role of Lin28B in the pathogenesis of EMT, and unraveled a novel mechanism that miR-26a is a modulator of let-7d. This study also defines the miRNAs network involved in IPF, which may have implications for developing new strategies for pulmonary fibrosis.
Key message
-
Upregulation of Lin28B contributes to idiopathic pulmonary fibrosis.
-
Lin28B causes epithelial-mesenchymal transition (EMT) by inhibition of let-7d.
-
Lin28B is one of the targets of microRNA-26a.
-
miR-26a enhances the expression of let-7d via targeting regulation of Lin28B.
-
A regulatory network among miRNAs involved in the progression of IPF.






Similar content being viewed by others
References
Yang IV, Schwartz DA (2015) Epigenetics of idiopathic pulmonary fibrosis. Transl Res 165:48–60
Chitra P, Saiprasad G, Manikandan R, Sudhandiran G (2015) Berberine inhibits Smad and non-Smad signaling cascades and enhances autophagy against pulmonary fibrosis. J Mol Med (Berl) 93:1015–1031
Ryu JH, Moua T, Daniels CE, Hartman TE, Yi ES, Utz JP, Limper AH (2014) Idiopathic pulmonary fibrosis: evolving concepts. Mayo Clin Proc 89:1130–1142
Liang H, Gu Y, Li T, Zhang Y, Huangfu L, Hu M, Zhao D, Chen Y, Liu S, Dong Y et al (2014) Integrated analyses identify the involvement of microRNA-26a in epithelial-mesenchymal transition during idiopathic pulmonary fibrosis. Cell Death Dis 5:e1238
Liang H, Xu C, Pan Z, Zhang Y, Xu Z, Chen Y, Li T, Li X, Liu Y, Huangfu L et al (2014) The antifibrotic effects and mechanisms of microRNA-26a action in idiopathic pulmonary fibrosis. Mol Ther 22:1122–1133
Oak SR, Murray L, Herath A, Sleeman M, Anderson I, Joshi AD, Coelho AL, Flaherty KR, Toews GB, Knight D et al (2011) A micro RNA processing defect in rapidly progressing idiopathic pulmonary fibrosis. PLoS One 6:e21253
Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E (2010) miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 207:1589–1597
van Rooij E (2011) The art of microRNA research. Circ Res 108:219–234
Pandit KV, Corcoran D, Yousef H, Yarlagadda M, Tzouvelekis A, Gibson KF, Konishi K, Yousem SA, Singh M, Handley D et al (2010) Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 182:220–229
Shyh-Chang N, Daley GQ (2013) Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell 12:395–406
Inui M, Martello G, Piccolo S (2010) MicroRNA control of signal transduction. Nat Rev Mol Cell Biol 11:252–263
Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv XB, Li F, Yu FX, Sun Y, Yuan H et al (2015) Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest 125:2123–2135
Chuquimia OD, Petursdottir DH, Rahman MJ, Hartl K, Singh M, Fernandez C (2012) The role of alveolar epithelial cells in initiating and shaping pulmonary immune responses: communication between innate and adaptive immune systems. PLoS One 7:e32125
Liang H, Li X, Wang L, Yu S, Xu Z, Gu Y, Pan Z, Li T, Hu M, Cui H et al (2013) MicroRNAs contribute to promyelocyte apoptosis in As2O3-treated APL cells. Cell Physiol Biochem 32:1818–1829
Liang H, Zhang C, Ban T, Liu Y, Mei L, Piao X, Zhao D, Lu Y, Chu W, Yang B (2012) A novel reciprocal loop between microRNA-21 and TGFbetaRIII is involved in cardiac fibrosis. Int J Biochem Cell Biol 44:2152–2160
Yang IV, Coldren CD, Leach SM, Seibold MA, Murphy E, Lin J, Rosen R, Neidermyer AJ, McKean DF, Groshong SD et al (2013) Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax 68:1114–1121
Moore BB, Hogaboam CM (2008) Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294:L152–L160
Xi YN, Xin XY, Ye HM (2014) Effects of HMGA2 on malignant degree, invasion, metastasis, proliferation and cellular morphology of ovarian cancer cells. Asian Pac J Trop Med 7:289–292
Huleihel L, Ben-Yehudah A, Milosevic J, Yu G, Pandit K, Sakamoto K, Yousef H, LeJeune M, Coon TA, Redinger CJ et al (2014) Let-7d microRNA affects mesenchymal phenotypic properties of lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 306:L534–L542
Desjardins A, Bouvette J, Legault P (2014) Stepwise assembly of multiple Lin28 proteins on the terminal loop of let-7 miRNA precursors. Nucleic Acids Res 42:4615–4628
Huang Y (2012) A mirror of two faces: Lin28 as a master regulator of both miRNA and mRNA. Wiley Interdiscip Rev RNA 3:483–494
Margadant C, Sonnenberg A (2010) Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep 11:97–105
Lopez-Novoa JM, Nieto MA (2009) Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 1:303–314
Radisky DC, Kenny PA, Bissell MJ (2007) Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem 101:830–839
Xiao J, Meng XM, Huang XR, Chung AC, Feng YL, Hui DS, Yu CM, Sung JJ, Lan HY (2012) miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice. Mol Ther 20:1251–1260
Yang S, Banerjee S, de Freitas A, Sanders YY, Ding Q, Matalon S, Thannickal VJ, Abraham E, Liu G (2012) Participation of miR-200 in pulmonary fibrosis. Am J Pathol 180:484–493
Zhao S, Ye X, Xiao L, Lian X, Feng Y, Li F, Li L (2014) MiR-26a inhibits prostate cancer progression by repression of Wnt5a. Tumour Biol 35:9725–9733
Schlosser K, White RJ, Stewart DJ (2013) miR-26a linked to pulmonary hypertension by global assessment of circulating extracellular microRNAs. Am J Respir Crit Care Med 188:1472–1475
Zhang ZH, Li J, Liu BR, Luo CF, Dong Q, Zhao LN, Zhong Y, Chen WY, Chen MS, Liu SM (2013) MicroRNA-26 was decreased in rat cardiac hypertrophy model and may be a promising therapeutic target. J Cardiovasc Pharmacol 62:312–319
Ambros V (2001) microRNAs: tiny regulators with great potential. Cell 107:823–826
Piskounova E, Viswanathan SR, Janas M, LaPierre RJ, Daley GQ, Sliz P, Gregory RI (2008) Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J Biol Chem 283:21310–21314
Viswanathan SR, Daley GQ, Gregory RI (2008) Selective blockade of microRNA processing by Lin28. Science 320:97–100
Davis BN, Hilyard AC, Lagna G, Hata A (2008) SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454:56–61
Zhong X, Chung AC, Chen HY, Meng XM, Lan HY (2011) Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol 22:1668–1681
Ahn SM, Cha JY, Kim J, Kim D, Trang HT, Kim YM, Cho YH, Park D, Hong S (2012) Smad3 regulates E-cadherin via miRNA-200 pathway. Oncogene 31:3051–3059
Viswanathan SR, Daley GQ (2010) Lin28: a microRNA regulator with a macro role. Cell 140:445–449
Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho J, Yeom KH, Han J, Kim VN (2009) TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138:696–708
Chen PS, Su JL, Cha ST, Tarn WY, Wang MY, Hsu HC, Lin MT, Chu CY, Hua KT, Chen CN et al (2011) miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J Clin Invest 121:3442–3455
Guo L, Sun B, Wu Q, Yang S, Chen F (2012) miRNA-miRNA interaction implicates for potential mutual regulatory pattern. Gene 511:187–194
Acknowledgments
This study was supported by National Basic Research Program of China (973 program, 2013CB531104); the Funds for Creative Research Groups of the National Natural Science Foundation of China (grant 81421063); the National Natural Science Foundation of China (31300943, 31450009); the Research Fund for the Doctoral Program of Higher Education of China (20132307110004); the Scientific Fund of Heilongjiang Province for Youth (QC2015100), and the Heilongjiang Postdoctoral Foundation (LBH-TZ0617).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
IPF lung tissue samples were obtained from surgical remnants of biopsies, and control samples were obtained from resected tissues from patients with lung cancer through the Second Affiliated Hospital of the Harbin Medical University under the procedures approved by the Ethics Committee for Use of Human Samples of Harbin Medical University (Harbin, China). Animals (8-week-old C57BL/6 mice; 20–30 g) used in this work were in accordance with the regulations of the Ethics Committees of Harbin Medical University and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Haihai Liang, Shanshan Liu and Yang Chen contributed equally to this work.
Additional file
Below is the link to the electronic supplementary material.
ESM 1
Electronic supplementary material. (PDF 257 kb)
Rights and permissions
About this article
Cite this article
Liang, H., Liu, S., Chen, Y. et al. miR-26a suppresses EMT by disrupting the Lin28B/let-7d axis: potential cross-talks among miRNAs in IPF. J Mol Med 94, 655–665 (2016). https://doi.org/10.1007/s00109-016-1381-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1381-8