Abstract
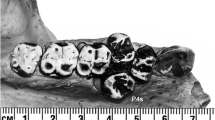
Appearance of the tribosphenic molar in the Late Jurassic (160 Ma) is a crucial innovation for food processing in mammalian evolution. This molar type is characterized by a protocone, a talonid basin and a two-phased chewing cycle, all of which are apomorphic. In this functional study on the teeth of Late Jurassic Dryolestes leiriensis and the living marsupial Monodelphis domestica, we demonstrate that pretribosphenic and tribosphenic molars show fundamental differences of food reduction strategies, representing a shift in dental function during the transition of tribosphenic mammals. By using the Occlusal Fingerprint Analyser (OFA), we simulated the chewing motions of the pretribosphenic Dryolestes that represents an evolutionary precursor condition to such tribosphenic mammals as Monodelphis. Animation of chewing path and detection of collisional contacts between virtual models of teeth suggests that Dryolestes differs from the classical two-phased chewing movement of tribosphenidans, due to the narrowing of the interdental space in cervical (crown–root transition) direction, the inclination angle of the hypoflexid groove, and the unicuspid talonid. The pretribosphenic chewing cycle is equivalent to phase I of the tribosphenic chewing cycle, but the former lacks phase II of the tribosphenic chewing. The new approach can analyze the chewing cycle of the jaw by using polygonal 3D models of tooth surfaces, in a way that is complementary to the electromyography and strain gauge studies of muscle function of living animals. The technique allows alignment and scaling of isolated fossil teeth and utilizes the wear facet orientation and striation of the teeth to reconstruct the chewing path of extinct mammals.





Similar content being viewed by others
References
Averianov A, Martin T, Lopatin A (2013) A new phylogeny for basal Trechnotheria and Cladotheria and affinities of South American endemic Late Cretaceous mammals. Naturwissenschaften 100:311–326
Benazzi S, Kullmer O, Grosse IR, Weber GW (2011) Using occlusal wear information and finite element analysis to investigate stress distributions in human molars. J Anat 219:259–272
Benazzi S, Kullmer O, Schulz D, Gruppioni G, Weber GW (2013) Technical note: individual tooth macrowear pattern guides the reconstruction of the Sts 52 (Australopithecus africanus) dental arches. Am J Phys Anthropol 150:324–329
Billet G, Blondel C, De Muizon C (2009) Dental microwear analysis of notungulates (Mammalia) from Salla (Late Oligocene, Bolivia) and discussion on their precocious hypsodonty. Palaeogeogr Palaeoclimatol Palaeoecol 274:114–124
Butler PM (1952) The milk-molars of Perissodactyla, with remarks on molar occlusion. Proc Zool Soc London 121:777–817
Butler PM (1972) Some functional aspects of molar evolution. Evolution 26:474–483
Butler PM (1988) Docodont molars as tribosphenic analogues. In: Russell DE, Santoro JP, Sigogneau-Russell D (eds) Teeth revisited: proceedings of the VIIth international symposium on dental morphology. Mémoires du Muséum National d’Histoire Naturelle, Séries C, 53:329–340
Chimento NR, Agnolin FL, Novas FE (2012) The Patagonian fossil mammal Necrolestes: a Neogene survivor of Dryolestoidea. Rev del Museo Argent de Ciencias Naturales 14:261–306
Chow M, Rich TH (1982) Shuotherium dongi, n. gen. and sp., a therian with pseudo-tribosphenic molars from the Jurassic of Sichuan. China Aust Mammal 5:127–142
Cifelli RL, Madsen SK (1999) Spalacotheriid symmetrodonts (Mammalia) from the medial Cretaceous (Upper Albian or lower Cenomnian) Mussentuchit local fauna, Cedar Mountain Formation, Utah, USA. Geodiversitas 21:167–214
Clauss M, Nunn C, Fritz J, Hummel J (2009) Evidence for a tradeoff between retention time and chewing efficiency in large mammalian herbivores. Comp Biochem Physiol A 154:376–382
Clemens WA (1970) Mesozoic mammalian evolution. Annu Rev Ecol Syst 1:357–390
Clemens WA, Kielan-Jaworowska Z (1979) Multituberculates. In: Lillegraven JA, Kielan-Jaworowska Z, Clemens WA (eds) Mesozoic mammals: the first two-thirds of mammalian history. University of California Press, Berkeley, pp 99–149
Cope ED (1883) On the trituberculate type of molar tooth in the mammalia. Proc Am Philos Soc 21:324–326
Crompton AW (1971) The origin of the tribosphenic molar. In: D. M. Kermack & K. A. Kermack (eds) Early mammals. Zoological Journal of the Linnean Society 50:65–87
Crompton AW, Hiiemäe K (1970) Molar occlusion and mandibular movements during occlusion in the American opossum, Didelphis marsupialis L. Zool J Linnean Soc 49:21–47
Crompton AW, Jenkins FA (1968) Molar occlusion in Late Triassic mammals. Biol Rev 43:427–458
Crompton AW, Luo Z-X (1993) Relationships of the Liassic Mammals Sinoconodon, Morganucodon oehleri, and Dinnetherium. In: Szalay F, Novacek MJ, McKenna MC (eds) Mammal phylogeny: Mesozoic differentiation, multituberculates, monotremes, Early Therians and marsupials. Springer-Verlag, New York, pp 30–44
Crompton AW, Sun A-L (1985) Cranial structure and relationsships of the Liassic mammal Sinoconodon. Zool J Linnean Soc 85:99–119
Crompton AW, Barnet J, Lieberman DE, Owerkowicz T, Skinner J, Baudinette RV (2008) Control of jaw movements in two species of macropodines (Macropus eugenii and Macropus rufus). Comp Biochem Physiol A 150:109–123
Davis BM (2011) Evolution of the tribosphenic molar pattern in early mammals, with comments on the “dual-origin” hypothesis. J Mamm Evol 18:227–244
Evans AR, Sanson GD (1998) The effect of tooth shape on the breakdown of insects. J Zool 246:391–400
Evans AR, Sanson GD (2005a) Biomechanical properties of insects in relation to insectivory: cuticule thickness as an indicator of insect ‘hardness’ and ‘intractability’. Aust J Zool 53:9–19
Evans AR, Sanson GD (2005b) Correspondence between tooth shape and dietry biomechanical properties in insectivorous microchiropterans. Evol Ecol Res 7:453–478
Evans AR, Wilson GP, Fortelius M, Jernvall J (2007) High-level similarity of dentitions in carnivorans and rodents. Nature 445:78–81
Flynn JJ, Parrish M, Rakotosamimanana B, Simpson WF, Wyss AR (1999) A Middle Jurassic mammal from Madagascar. Nature 401:57–60
Fortelius M, Solounias N (2000) Functional characterization of ungulate molars using the abrasion-attrition wear gradient: a new method for reconstructing paleodiets. Am Mus Novit 3301:1–36
Fraenkel G, Rudall KM (1940) A study of the physical and chemical properties of the insect cuticle. Proc R Soc Lond B 129:1–35
Freeman PW (1979) Specialized insectivory: beetle-eating and moth-eating molossid bats. J Mammal 60:467–479
Freeman PW (1981) Correspondence of food habits and morphology in insectivorous bats. J Mammal 62:164–166
Fritz J, Hummel J, Kienzle E, Arnold C, Nunn C, Clauss M (2009) Comparative chewing efficiency in mammalian herbivores. Oikos 118:1623–1632
Gordon KD (1984) The assessment of jaw movement direction from dental microwear. Am J Phys Anthropol 63:77–84
Gurovich Y (2005) Bio-Evolutionary aspects of Mesozoic mammals: description, phylogenetic relationships and evolution of the Gondwanatheria, (Late Cretaceous and Paleocene of Gondwana). Thesis, Universidad de Buenos Aires
Gurovich Y, Beck R (2009) The phylogenetic affinities of the enigmatic mammlian clade Gondwanatheria. J Mamm Evol 16:25–49
Hahn G (1969) Beiträge zur Fauna der Grube Guimarota. Nr. 3: Die Multituberculata. Palaeontogr Abt A 133:1–100
Hiiemäe KM (1978) Mammalian mastication: a review of the activity of the jaw muscles and the movements they produce in chewing. In: Butler PM, Joysey KA (eds) Development, function and evolution of teeth. Academic Press, London, pp 359–398
Hiiemäe KM (1984) Functional aspects of primate jaw morphology. In: Chivers DJ, Hladik CH (eds) Food acquisition and processing in nonhuman primates. Academic Press, London, pp 257–281
Hiiemäe K, Kay RF (1972) Trends in the evolution of primate mastication. Nature 240:486–487
Hiiemäe KM, Kay RF (1973) Evolutionary trends in the dynamics of primate mastication. In: Montagna W, Zingeser MR (eds) Symposia of the international congress of primatology, vol 3. Karger, Basel, pp 28–64
Hunter JP, Fortelius M (1994) Comparative dental occlusal morphology, facet development, and microwear in two sympatric species of Listriodon (Mammalia: Suidae) from the Middle Miocene of Western Anatolia (Turkey). J Vertebr Paleontol 14:105–126
Hylander WL, Johnson KR, Crompton AW (1987) Loading patterns and jaw movements during mastication in Macaca fascicularis: a bone-strain, electromyographic, and cineradiographic analysis. Am J Phys Anthropol 72:287–314
Ji Q, Luo Z-X, Yuan C-X, Tabrum AR (2006) A swimming mammaliaform from the Middle Jurassic and ecomorphological diversification of early mammals. Science 311:1123–1127
Kaiser T, Fortelius M (2003) Differential mesowear in occluding upper and lower molars: opening mesowear analysis for lower molars and premolars in hypsodont horses. J Morphol 258:67–83
Kaiser T, Solounias N (2003) Extending the tooth mesowear method to extinct and extant equids. Geodiversitas 25:341–345
Kemp TS (1982) Mammal-like reptiles and the origin of mammals. Academic Press, London
Kielan-Jaworowska Z, Cifelli RL, Luo Z-X (2002) Dentition and relationships of the Jurassic mammal Shuotherium. Acta Palaeontol Pol 47:479–486
Kielan-Jaworowska Z, Cifelli RL, Luo Z-X (2004) Mammals from the age of dinosaurs: origins, evolution and structure. Columbia University Press, New York
Krause DW (1982) Jaw movement, dental function, and diet in the paleocene multituberculate Ptilodus. Paleobiology 8:265–281
Kullmer O, Benazzi S, Fiorenza L, Schulz D, Bacso S, Winzen O (2009) Technical note: occlusal fingerprint analysis: quantification of tooth wear pattern. Am J Phys Anthropol 139:600–605
Kullmer O, Benazzi S, Schulz D, Gunz P, Kordos L, Begun DR (2013) Dental arch restoration using tooth macrowear patterns with application to Rudapithecus hungaricus, from the late Miocene of Rudabánya. Hung J Hum Evol 64:151–160
Lazzari V, Schultz JA, Tafforeau P, Martin T (2010) Occlusal pattern in paulchoffatiid multituberculates and the evolution of cusp morphology in mammaliamorphs with rodent-like dentitions. J Mamm Evol 17:177–192
Lieberman DE, Crompton AW (2000) Why fuse the mandibular symphysis? A comparative analysis. Am J Phys Anthropol 112:517–540
Lucas PW (1979) The dental-dietary adaptations of mammals. Neues Jb Geol Paläontol Monat 8:486–512
Lucas PW et al (2013) Mechanisms and causes of wear in tooth enamel: implications for hominin diets. J R Soc Interface 10:20120923
Luo Z-X (2007) Transformation and diversification in early mammal evolution. Nature 450:1011–1019
Luo Z-X, Wible JR (2005) A Late Jurassic digging mammal and early mammalian diversification. Science 308:103–107
Luo Z-X, Cifelli RL, Kielan-Jaworowska Z (2001) Dual origin of tribosphenic mammals. Nature 409:53–57
Luo Z-X, Kielan-Jaworowska Z, Cifelli RL (2002) In quest for a phylogeny of mesozoic mammals. Acta Palaeontol Pol 47:1–78
Luo Z-X, Ji Q, Yuan C-X (2007) Convergent dental adaptions in pseudo-tribosphenic mammals and tribosphenic mammals. Nature 450:93–97
Luo Z-X, Yuan C-X, Meng Q-J, Ji Q (2011) A Jurassic eutherian mammal and divergence of marsupials and palcentals. Nature 476:442–445
Luschei ES, Goodwin GM (1974) Patterns of mandibular movement and jaw muscle activity during mastication in the monkey. J Neurophysiol 37:954–966
Maier W (1980) Konstruktionsmorphologische Untersuchungen am Gebiß der rezenten Prosimiae (Primates). Abh Senckenb Naturforsch Ges 538:1–158
Martin T (1999) Dryolestidae (Dryolestoidea, Mammalia) aus dem Oberen Jura von Portugal. Abh Senckenb Naturforsch Ges 550:1–119
Martin T (2005) Postcranial anatomy of Haldanodon exspectatus (Mammalia, Docodonta) from the Late Jurassic (Kimmeridgian) of Portugal and its bearing for mammalian evolution. Zool J Linnean Soc 145:219–248
Martin T (2006) Early mammalian evolutionary experiments. Science 311:1109–1110
Meng J, Hu Y, Wang Y, Wang X, Li C (2006) A mesozoic gliding mammal from northeast China. Nature 444:889–893
Mihlbachler MC, Rivals F, Solounias N, Semprebon GM (2011) Dietary change and evolution of horses in North America. Science 331:1178–1181
Moore SJ, Sanson GD (1995) A comparison of the molar efficiency of two insect-eating mammals. J Zool 235:175–192
Osborn HF (1888) The evolution of mammalian molars to and from the tritubercular type. Am Nat 22:1067–1079
Pfretzschner H-U, Martin T, Maisch MW, Matzke AT, Sun G (2005) A new docodont mammal from the Late Jurassic of the Junggar Basin in Northwest China. Acta Palaeontol Pol 50:799–808
Prothero DR (1981) New Jurassic mammals from Como Bluff, Wyoming, and the interrelationships of non-tribosphenic theria. Bull Am Mus Nat Hist 167:277–326
Rivals F, Semprebon GM (2011) Dietary plasticity in ungulates: insight from tooth microwear analysis. Quartern Int 245:279–284
Rougier GW, Apesteguía S, Gaetano L (2011) Highly specialized mammalian skulls from the Late Cretaceous of South America. Nature 479:98–102
Rougier GW, Wible JR, Beck RM, Apesteguia S (2012) The Miocene mammal Necrolestes demonstrates the survival of a Mesozoic nontherian lineage into the late Cenozoic of South America. Proc Natl Acad Sci U S A 109:20053–20058
Santana SE, Strait S, Dumont ER (2011) The better to eat you with: functional correlates of tooth structures in bats functional. Ecology 25:839–847
Schultz JA (2012) Funktionelle Morphologie und Abnutzungsmuster prätribosphenischer Molaren am Beispiel der Dryolestida (Mammalia, Cladotheria). Thesis, Rheinische Friedrich-Wilhelms-Universität Bonn
Schultz JA, Martin T (2011) Wear pattern and functional morphology of dryolestoid molars (Mammalia, Cladotheria). Paläontol Z 85:269–285
Schulz E, Calandra I, Kaiser T (2010) Applying tribology to teeth of hoofed mammals. Scanning 32:162–182
Scott R, Ungar PS, Bergstrom TS, Brown CA, Grine FE, Teaford MF, Walker A (2005) Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature 436:693–695
Sheine WS, Kay RF (1977) An analysis of chewed food particle size and its relationship to molar sturcture in the primates Cheirogaleus medius and the Galago senegalensis and the insectivoran Tupaia glis. Am J Phys Anthropol 47:15–20
Sibbing FA (1991) Mastication in cyprinid fish. In: Vincent JFV, Lillefort P (eds) Feeding and the texture of food. Symp Soc Exp Biol, 43:57–92
Spoutil F, Vlcek V, Horacek I (2010) Enamel microarchitecture of a tribosphenic molar. J Morphol 271:1204–1218
Teaford MF (1988) A review of dental microwear and diet in modern mammals. Scanning Microsc 2:1149–1166
Ungar PS, Williamson M (2000) Exploring the effects of toothwear on functional morphology: a preliminary study using dental topographic analysis. Palaeontol Electron 3:1–18
Ungar PS, Bergstrom TS, Walker A (2003) Quantification of dental microwear by tandem scanning confocal microscopy and scale-sensitive fractal analysis. Scanning 25:185–193
Walker A, Hoeck HN, Perez L (1978) Microwear of mammalian teeth as an indicator of diet. Science 201:908–910
Wall CE, Vinyard CJ, Johnson KR, Willimas SH, Hylander WL (2006) Phase II jaw movements and masseter muscle activity during chewing in Papio anubis. Am J Phys Anthropol 129:215–224
Wang Y-Q, Clemens WA, Hu Y-M, Li C-K (1998) A probable pseudo-tribosphenic upper molar from the Late Jurasic of China and the early radiation of the holotheria. J Vertebr Paleontol 18:777–787
Weijs WA, Dantuma R (1975) Electromyography and mechanics of mastication in the albino rat. J Morphol 146:1–34
Wilson GP, Evans AR, Corfe IJ, Smits PD, Fortelius M, Jernvall J (2012) Adaptive radiation of multituberculate mammals before the extinction of dinosaurs. Nature 483:457–460
Woodburne MO (2003) Monotremes as pretribosphenic mammals. J Mamm Evol 10:195–248
Zheng X, Bi S, Wang X, Meng J (2013) A new arboreal haramiyid shows the diversity of crown mammals in the Jurassic period. Nature 500:199–202
Zhou C-F, Wu S, Martin T, Luo Z-X (2013) A Jurassic mammaliaform and the earliest mammalian evolutionary adaptations. Nature 500:163–167
Acknowledgments
We thank Z.-X. Luo, T. Smith, G W. Rougier and two anonymous reviewers for their critical comments on the manuscript. This study is funded by the Deutsche Forschungsgemeinschaft (DFG) as part of the DFG research unit 771 “Function and performance enhancement in the mammalian dentition” (project D1; MA 1643/16-1) and is publication number 67 of the DFG research unit 771. We thank the members of the DFG research unit 771 for fruitful discussions, especially O. Kullmer (Senckenberg Forschungsinstitut und Naturmuseum Frankfurt, Germany) and colleagues (ZiLoX IT) for support and advice for the OFA software. G. Wilson helped to improve an older version of the manuscript. The dryolestid material was collected during field operations under the supervision of S. Henkel and B. Krebs of the Freie Universität Berlin (Germany). Fieldwork in Portugal was supported by the Serviços Geológicos de Portugal (Lisbon). A. H. Schwermann (Universität Bonn, Germany) shared polygonal models of Monodelphis domestica. We thank R. Schellhorn for discussion and D. Kranz (both from Universität Bonn, Germany) for suggestions that improved the artwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Electronic supplementary material
Below is the link to the electronic supplementary material.
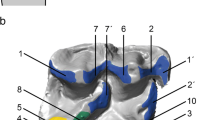
During the repeated chewing movement of Dryolestes leiriensis, the occlusion of the paracone into the interdental space between two lower adjacent teeth is obvious. When the lower teeth are lifted, the paracone slides in to the interdental space and touches the hypoflexid groove lingually then slides down the groove in buccal direction. Interdental shearing is demonstrated by the collisional contacts that are detected between the polygonal surface models during every time step of the animated movement. The detected collisional contacts between the upper and lower teeth are shown in gradient colors (collisions of three time steps overlaid in one time step, green to red) in the first seven chewing cycles. To fully visualize all collisions that occur during the chewing movement, the upper molar is animated to be translucent after one cycle and the scene is changing from anterior view to buccal view and to occlusal view. In chewing cycle 8 (minute 1:32), the collisions are shown in normal coloring without overlay, for the viewer to get an impression of the size and allocation of detected areas between the polygonal models. The orange line is the path of the lower jaw calculated by the OFA. (MPG 9356 kb)
The animated chewing cycle of Dryolestes leiriensis in occlusal view with a translucent upper molar. The upper molar is animated to disappear after three chewing cycles for better view of the detected collisions in the interdental space between the lower molars. The visualization of the collisions starts with gradient colors (collisions of three time steps overlaid in one, green to red), and then changes in the third chewing cycle to normal coloring of the detected areas. The orange line is the path of the lower jaw calculated by the OFA. (MPG 5216 kb)
During the repeated chewing movement of Monodelphis domestica the occlusion of the protocone into the talonid basin positioned between the trigonids of two lower adjacent molars is obvious. When the lower teeth are lifted, the protocone first slides in to the talonid basin lingually then crosses the deepest part of the basin in direction of the hypoconid. The shearing is demonstrated by the collisional contacts that are detected between the polygonal surface models along the nearly vertical aspects of the trigon and the two trigonids during every time step of the animated movement. Crushing occurs when the protocone crossed the horizontal floor of the talonid basin. Leaving the talonid basin in buccal direction, the protocone crosses the lingual side of the hypoconid. Here, the additional collisional contacts are evident, which are nonexistent in Dryolestes leiriensis. The change of direction in the chewing path from downward to upward after crossing the deepest part of the talonid basin depicts the transition from phases I to II. The detected collisional contacts between the upper and lower teeth are shown in gradient colors (collisions of three time steps overlaid in one timestep, green to red) in the first seven chewing cycles. To fully visualize, all collisions that occur during the chewing movement the upper molar is animated to be translucent after one cycle and the scene is changing from anterior view to buccal view and to occlusal view. In chewing cycle 7 (minute 1:43), the collisions are shown in normal coloring without overlay, for the viewer to get an impression of the size and allocation of detected areas between the polygonal models. The orange line is the path of the lower jaw calculated by the OFA. (MPG 14582 kb)
The animated chewing cycle of Monodelphis domestica in occlusal view with a translucent upper molar. The upper molar is animated to disappear after three chewing cycles for better view of the detected collisions in the talonid basin between the lower molars. The visualization of the collisions starts with gradient colors (collisions of three time steps overlaid in one, green to red) and then changes in the third chewing cycle to normal coloring of the detected areas. The orange line is the path of the lower jaw calculated by the OFA. (MPG 8630 kb)
ESM 5
(DOCX 434 kb)
Rights and permissions
About this article
Cite this article
Schultz, J.A., Martin, T. Function of pretribosphenic and tribosphenic mammalian molars inferred from 3D animation. Naturwissenschaften 101, 771–781 (2014). https://doi.org/10.1007/s00114-014-1214-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-014-1214-y