Abstract
Purpose
The Doppler-based resistive index (RI) and semi-quantitative evaluation of renal perfusion using color Doppler (SQP) have shown promising results for predicting persistent acute kidney injury (AKI) in preliminary studies. This study aimed at evaluating the performance of RI and SQP to predict short-term renal prognosis in critically ill patients.
Methods
Prospective multicenter cohort study including unselected critically ill patients. Renal Doppler was performed at admission to the intensive care unit. The diagnostic performance of RI and SQP to predict persistent AKI at day 3 was evaluated.
Results
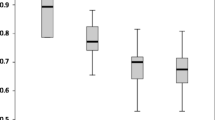
Overall, 371 patients were included, of whom 351 could be assessed for short-term renal recovery. Two thirds of the included patients had AKI (n = 233; 66.3%), of whom 136 had persistent AKI (58.4%). Doppler-based RI was higher and SQP lower in AKI patients and according to AKI recovery. Overall performance in predicting persistent AKI was however poor with area under ROC curve of respectively 0.58 (95% CI 0.52–0.64) and 0.59 (95% CI 0.54–0.65) for RI and SQP. Optimal cutoff was respectively 0.71 and 2 for RI and SQP. At optimal cutoff, sensitivity and specificity were 50% (95% CI 41–58%) and 68% (62–74%) for RI and 39% (32–45%) and 75% (66–82%) for SQP.
Conclusion
Although statistically associated with AKI occurrence, RI and SQP perform poorly in predicting persistent AKI at day 3. Further studies are needed to adequately describe factors influencing Doppler-based assessment of renal perfusion and to delineate whether these indicators may be useful at the bedside.
Clinicaltrial.gov
NCT02355314.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is common in intensive care unit (ICU) patients and remains associated with a dismal prognosis [1,2,3]. Prompt diagnosis of AKI is hampered by several important limitations of its usual markers [4]. Oliguria is unspecific, and serum creatinine elevation is delayed and only occurs after a prolonged decrease in the glomerular filtration rate [1, 5]. These limits urge KDIGO experts to recommend validation of an alternate marker allowing the early diagnosis, differential diagnosis, or prognostic evaluation of AKI [6].
Doppler-based renal resistive index (RI) measurement is a rapid and non-invasive tool that was proposed for early AKI detection [7,8,9] or to differentiate transient from persistent AKI in ICU patients [7, 9, 10]. Semi-quantitative evaluation of renal perfusion (SQP) using color Doppler has also been proposed as a simple tool and showed similar performance [11]. Although suggesting promising performance of these tools to predict short-term prognosis of AKI, most of these studies were performed in an expert center with limited patient samples [7, 9, 12, 13]. A recent meta-analysis confirmed this diagnostic performance but also reported a high heterogeneity between studies [14]. Actually, these results must be tempered by the high risk of bias associated with these preliminary studies and the numerous confounding factors known to influence RI [15, 16]. Indeed, discrepant studies showed limited diagnostic performance of RI [12, 17].
Therefore, a multicenter prospective cohort study was undertaken to evaluate the performance of Doppler-based RI and SQP to predict persistent AKI in a large cohort of unselected critically ill patients. The secondary objective was to assess the performance of these tools to predict the need for renal replacement therapy (RRT).
Methods
Patients
The study was approved by the Saint-Etienne institutional review board. Patients and their next of kin were informed, and informed consent was obtained before inclusion. The study protocol was registered in clinicaltrial.gov (NCT02355314).
Seven university hospital ICUs and one community-based hospital ICU in France participated in the study between June 2013 and June 2016. Consecutive adults admitted to these ICUs and requiring mechanical ventilation were included. Patients with obstructive renal disease, underlying severe chronic kidney disease (stage IV or V), with previously known renal artery stenosis or renal vein thrombosis, with cardiac arrhythmia precluding renal Doppler measurement, persons deprived of liberty or under guardianship or curators, patients with expected hospital stay < 72 h, patients with AKI having already recovered according to our definition, and pregnant patients were not included. Last, patients with hospital stays < 72 h, since they could not be classified as having transient or persistent AKI according to our definitions, were secondarily excluded. Screening was ongoing as long as two investigators trained in Doppler were available.
Although the study was open, investigators were blinded to the primary objective of this study.
Definitions
Acute kidney injury was defined as any degree of AKI at study inclusion according to the KDIGO definition (plasma creatinine level increase of 26.4 µmol/l or more, plasma creatinine increase ≥ 150% from baseline, or urine output < 0.5 ml/kg/h for 6 h or more) [18]. For patients whose baseline plasma creatinine level was unknown, this variable was estimated according to Modification of Diet in Renal Disease (MDRD) formula back-calculation [18]. AKI severity was defined according to the KDIGO definition [18].
Transient AKI was defined as AKI with recovery occurring before H72 [7, 19, 20]. Short-term renal recovery was defined as a decrease of at least one stage in AKI severity according to the KDIGO criteria (i.e., a decrease of serum creatinine, reversal of oliguria in the absence of diuretic therapy, and absence of RRT requirement) [7]. Persistent AKI was defined by any degree of AKI not meeting the criteria for transient AKI. Diuretic use was defined as the use of diuretics at any time during the first 24 h in the ICU.
The Logistic Organ Dysfunction (LOD) score was calculated at study inclusion [21] and the Knaus scale score at ICU admission [22].
Sepsis and septic shock were diagnosed using the criteria developed at the American College of Chest Physicians/Society of Critical Care Medicine consensus conference [23].
Estimation of short-term renal recovery was performed by the senior attending physician, uninvolved in Doppler measurement. This estimation was the “estimation of probability of remaining free of AKI or of experiencing short-term renal recovery within 72 h” and was assessed by a Likert scale ranging from 0 to 100%.
Study protocol, data collection, and resistive index (RI) measurement
Assessments of renal perfusion and function as well as estimation of the probability of short-term renal recovery were performed concomitantly at study inclusion.
In line with already published methodology, during each study period, 27 investigators (all certified physicians) attended a half-day training session on Doppler evaluation of renal perfusion. After a discussion of relevant theoretical considerations, practical training was provided in locating the kidneys, identifying the intrarenal vessels using color Doppler, grading renal perfusion using the previously published semi-quantitative color Doppler scale, and measuring the RI [11]. Feasibility and reliability of both Doppler-based RI and SQP were assessed and found to be adequate [11].
The following clinical variables were collected from each patient before the measurement session: heart rate; systolic, diastolic, and mean blood pressures; urine output; respiratory rate; tidal volume; positive end-expiratory pressure; inspired fraction of oxygen; plateau pressure; type and dose of sedative; and type and dose of vasoactive drugs.
In each patient, renal sonography for RI measurement was performed at study inclusion by a physician not involved in patient management. After kidney visualization in grayscale and color Doppler modes, the absence of signs of chronic renal damage was checked. Renal perfusion was assessed by color Doppler using the semi-quantitative scale (Table S1) [11]. An interlobar or arcuate artery was then selected, and measurements were obtained using pulse-wave Doppler. The Doppler gain was set to obtain a clear outline of flow waves with minimal background noise. The Doppler spectrum was considered optimal when at least three similar consecutive waveforms were visualized. The resistive index was calculated as (peak systolic velocity − end diastolic velocity)/peak systolic velocity. The mean of three to five RI values was computed.
Statistical analysis
Data are described as median and interquartile range (IQR) or number and percentage. Categorical variables were compared using Fisher’s exact test and continuous variables using the nonparametric Wilcoxon test, Mann-Whitney test, or Kruskal-Wallis test. The Friedman test was used to compare continuous variables across the three patient groups (no AKI, transient AKI, and persistent AKI).
Based on previous findings, we estimated our sample size as follows [7, 19]. Four hundred patients would be needed to obtain 100 patients without AKI, 100 with transient AKI, and 100 persistent AKI, taking into account a loss to follow-up rate of 10% and a rate of failure to obtain Doppler-based RI of 10%. This would allow:
-
Detecting an absolute change > 0.03 in RI between groups with a statistical power of 0.95, alpha risk of 0.05, assuming an RI of 0.67 in patients without AKI [7].
-
Delineating diagnostic test performance with CI of ± 0.05 assuming an area under the receiver-operating characteristic (AUROC) curve of 0.80 [14, 24].
-
Assessing factors independently associated with persistent AKI, adjusting for nine variables in addition to RI or SQP [25].
Assessing diagnostic performance of the Doppler-based resistive index and semi-quantitative renal perfusion over the first 24 h in predicting persistent AKI, we plotted the receiver-operating characteristic (ROC) curves of the proportion of true positives against the proportion of false positives to classify patients as having persistent AKI. The confidence interval of the AUC was calculated and AUROC curves compared according to the DeLong method [26, 27]. The sensitivity, specificity, and confidence interval were approximated using bootstrapping methods [27, 28]. The optimal cutoff point was defined according to the optimal Youden’s J statistic [29].
Independent predictors of persistent AKI and need for RRT were assessed using logistic regression and mixed logistic models. First, a logistic regression model was built. Variables of interest were selected according to their relevance and statistical significance in univariate analysis. We used conditional stepwise regression with 0.2 as the critical P value for entry into the model and 0.1 as the P value for removal. It was planned a priori to force semi-quantitative perfusion or RI in the model should these variables not be selected. Interactions and correlations between the explanatory variables were carefully checked. Continuous variables for which log linearity was not confirmed were transformed into categorical variables according to the median or IQR. Renal perfusion indices being strongly correlated with AKI severity (AKI stage), the latter was not inserted in the final model.
Last, a mixed model was performed using previously selected variables using center as random effect. This model adjusting for center effect is reported in the article. All models were assessed for calibration, discrimination, and relevancy. Residuals were plotted and the distributions inspected.
In a post hoc analysis, performance of perfusion indices was tested in subgroups (namely patients with AKI defined by serum creatinine, patients with AKI defined by oliguria, and patients without missing baseline serum creatinine) to ensure homogeneity of the results. Criteria to define AKI were found to be associated with the AKI severity and rate of short-term renal recovery [19, 30]. This sensitivity analysis therefore aimed to assess consistency of the results in groups considered to have different risks of short-term renal recovery.
All tests were two sided, and P values < 0.05 were considered statistically significant. Analyses were done using R software version 3.4.4 (https://www.r-project.org), including the pROC, lme4, and lmerTest packages.
Results
Overall, 916 patients were screened, of whom 371 were ultimately included within 24 h of ICU admission (1 day, IQR 0–1). Main reasons for non-inclusion were cardiac arrhythmia and expected hospital stay < 72 h (Fig. 1).
Study population
The main characteristics of the study population are reported in Table 1. Overall, 371 patients were included, of whom 20 were secondarily excluded as non-evaluable at H72. Of the 351 patients admitted, two thirds had AKI according to the KDIGO definition (n = 233; 66.3%). Overall severity was AKI stage 1 in 149 patients (64.9%), stage 2 in 33 patients (14.2%), and stage 3 in 51 patients (21.9%). Criteria to define AKI were diuresis only in 135 patients, serum creatinine only in 30 patients, and presence of both criteria in 68 patients. Of the included patients with AKI, 97 recovered within 72 h and were classified as having transient AKI (41.6%) and 136 had a persistent AKI (58.4%). Older age, underlying comorbidities, illness severity as assessed by the LOD score, septic shock, emergency surgical status, hypovolemia, and number of nephrotoxic agents were associated with AKI and persistent AKI (Table 1).
Performance of the Doppler-based resistive index (RI) and semi-quantitative perfusion (SQP) in predicting persistent AKI
Doppler-based RI and SQP were obtained in all of the patients.
The resistive index was higher, and SQP lower, in AKI patients and according to short-term renal recovery (Table 1; Figs. 2a, b, 3a, b).
Overall performance in predicting persistent AKI was however poor with an area under the ROC curve of 0.58 (95% CI 0.52–0.64) and 0.59 (95% CI 0.54–0.65) for Doppler-based RI and SQP, respectively (Table 2; Fig. 4a, b). The optimal cutoff was respectively 0.71 and 2 for RI and SQP. At optimal cutoff, sensitivity and specificity were 50% (95% CI 41–58%) and 68% (62–74%) for RI and 39% (32–45%) and 75% (66–82%) for SQP (Table 2).
Before adjustment, SQP was associated with persistent AKI (OR 0.63 per point; 95% CI 0.47–0.85). After adjustment for confounders, logistic regression identified SQP as associated independently with persistent AKI (OR 0.69 per point; 95% CI 0.50–0.95).
Before adjustment, RI was associated with persistent AKI (OR 12.41 per 0.01 unit; 95% CI 1.65–98.25). When included in the stepwise selection, Doppler-based RI was not selected in the final model. When forced, RI was not associated with persistent AKI and did not change the model (OR 2.52 per 0.01 unit; 95% CI 0.27–22.83).
To assess a potential center effect, a sensitivity analysis was performed replicating multivariable analysis using a mixed model with center included as random effect. This analysis did not change the model, impact of selected variables, or impact of SQP or RI (Table 3).
Performance of Doppler-based resistive index (RI) and semi-quantitative perfusion (SQP) in predicting need for renal replacement therapy at day 3
Overall, 36 patients (10.3%) required RRT at day 3 (Table S2).
The resistive index was higher, and SQP lower, in patients requiring RRT (Table S2).
Overall performance in predicting need for RRT was however poor with an area under the ROC curve of respectively 0.65 (95% CI 0.57–0.73) and 0.66 (95% CI 0.58–0.75) for Doppler-based RI and SQP (Figure S1 and S2).
The optimal cutoff was respectively 0.74 and 2 for RI and SQP. At optimal cutoff, sensitivity and specificity were 74% (95% CI 69–79%) and 51% (34–68%) for RI and 41% (25–58%) and 81% (76–85%) for SQP.
Before adjustment, SQP was associated with the need for RRT (OR 0.43 per point; 95% CI 0.26–0.68). After adjustment for confounders, logistic regression identified SQP as associated independently with the need for RRT (OR 0.56 per point; 95% CI 0.33–0.93) along with illness severity as assessed by the norepinephrine dose (OR 4.15 per µg/kg/min; 95% CI 2.02–9.43) and FiO2 level (1.03 per %; 95% CI 1.01–1.04).
Before adjustment, RI was associated with the need for RRT (OR 74.04 per point; 95% CI 3.22–1743). When included in the stepwise selection, Doppler-based RI was not selected in the final model, and, when forced, RI was not associated with the need for RRT (OR 17.51; 95% CI 0.51–608) and did not change the model (Table S3).
To assess a potential effect of center effect, a sensitivity analysis was performed replicating multivariable analysis using a mixed model with center included as random effect. This analysis did not change the model, impact of selected variables, or impact of SQP or RI.
Accuracy of physician prediction of short-term renal recovery or need for renal replacement therapy
Physician estimation of probability for short-term renal recovery at study inclusion had similar performance in predicting persistent AKI (AUC ROC 0.59; 95% CI 0.52–0.65) or need for RRT (AUC ROC 0.76; 95% CI 0.66–0.85) than both Doppler-based perfusion estimators (Figure S2a and S2b).
Sensitivity analysis according to criteria defining AKI and availability of baseline serum creatinine
Performance of the perfusion index in predicting persistent AKI was not different in patients with AKI defined by serum creatinine, in patients with AKI defined by oliguria, and in patients without missing baseline serum creatinine (Table S4).
Discussion
This multicenter study is the largest to date assessing performance of Doppler-based RI and SQP in predicting persistent AKI and need for RRT. According to our results, neither Doppler-based RI nor SQP is useful in predicting persistent AKI. Although they might be able to predict need for RRT, performance in this regard is also poor.
Limits of usual markers of renal dysfunction, namely the insensitive serum creatinine [6] or the unspecific oliguria [1], urge KDIGO experts to recommend validation of a marker other than serum creatinine allowing the early diagnosis, differential diagnosis, or prognostic evaluation of AKI [18, 20]. Several studies suggested renal Doppler might help in predicting development of renal dysfunction [8] or short-term renal prognosis [14]. Although several preliminary studies conducted in the 1990s suggested that RI might help to separate transient from persistent AKI [10, 31, 32], these studies included limited numbers of patients, and both patient selection and definitions of transient and persistent AKI are unclear in most of these studies. In recent studies focusing on critically ill patients, RI measured at admission was significantly higher in patients with persistent or worsening of AKI [7, 9, 11]. In these studies, RI performed better than most of the urinary indices including urinary cystatin C [7, 9, 11]. A recent meta-analysis suggested a very good performance of renal Doppler in predicting short-term reversibility [14]. Several limits were however noted to these positive findings. First, discordant results were noted, with some studies reporting limited or poor performance of RI [12, 17]. Furthermore, most of the studies performed were at high risk of bias; numerous confounding factors have been described to influence RI, explaining the high heterogeneity noted among results of studies performed so far [14,15,16].
In addition to clinical data, several experimental studies suggested vast heterogeneity and inadequate comprehension of RI significance. RI has been assumed to faithfully reflect renal vascular resistance [33, 34]. In experimental studies, however, the association between Doppler-based RI and renal vascular resistance was weak. Large, non-physiologic, pharmacologically induced changes in renal vascular resistance translated into only small RI changes [33]. In addition, several intrarenal or systemic hemodynamic factors have been demonstrated to influence both RI and the association between RI and renal vascular resistance. In several studies, arterial stiffness (vascular compliance) was a major determinant of RI [33,34,35]. In addition, the relationship between vascular resistance and RI became weaker as arterial stiffness increased [34]. Furthermore, increases in interstitial or intraabdominal pressure reduce the transmural pressure of the renal arterioles, thereby diminishing arterial distension, decreasing vascular compliance and ultimately increasing RI [35]. Last, several hemodynamic factors including the pulse pressure index, mean arterial pressure, and heart rate have direct and dramatic effects on RI values [7, 8, 33, 34, 36]. Each of these factors may act as a confounder. The numerous factors involved in RI changes, along with the strong influence of preexisting vascular stiffness, have led to the hypothesis that RI might reflect renal and vascular history and therefore explain the previously demonstrated association between RI and the renal prognosis or pathologic mechanism of renal dysfunction [14, 37].
This study has several strengths. First, the multicenter design, large sample size, and consecutive inclusions may be expected to limit biases in assessing diagnostic test performance. The definition of persistent AKI was demonstrated as associated with relevant clinical end points, and training in renal Doppler was validated previously by our research group [11, 19]. Last, although negative, we ensured that this study had statistical power to detect relevant differences in RI between transient and persistent AKI and would have sufficient precision to allow a definite answer on this topic.
Our study also has limitations that may deserve to be discussed. First, our definition of transient AKI significantly differs from the recently suggested definition of the ADQI group [20]. However, this study was initiated before publication of this definition. In addition, the chosen definition of persistent AKI was previously shown to be associated with relevant clinical end points [19]. Although no definite blinding was possible, we made efforts to limit biases by defining recovery objectively, ensuring renal Doppler was performed by a physician uninvolved in patient care and blinding clinicians to study objectives. In this line, indications for RRT were not standardized, which may have influenced our findings. However, attending physicians were not informed of the Doppler measurement results, investigators were uninvolved in patient care, and the clustering effect related to local practices should have been partly adjusted for by the mixed model adjusted for center effect. Moreover, this study was designed to assess discrimination of the tested marker. The poor discrimination observed in this study does not preclude potential influence of these markers in allowing risk stratification [38, 39]. Future studies may be needed to assess the usefulness of these tests in this regard. Last, lack of experience of some operators may have contributed to the negative results of this study. However, when adjusting for center effect, including expertise as part of the variability of results, an association between RI or SQP and persistent AKI was not modified, suggesting lack of center effect.
In conclusion, this multicenter study suggests measurement of Doppler-based RI and semi-quantitative renal perfusion to be feasible at bedside. Although statistically associated with AKI occurrence and persistence, these indicators perform poorly in predicting persistent AKI at day 3 or need for RRT. Further studies are needed to adequately describe factors influencing Doppler-based assessment of renal perfusion and to delineate whether these indicators may be useful at bedside. Meanwhile, our results suggest neither Doppler-based RI nor semi-quantitative renal perfusion should be used to assess short-term renal prognosis in the ICU setting.
Abbreviations
- AKI:
-
Acute kidney injury
- AUROC curve:
-
Area under the receiver-operating characteristic curve
- CI:
-
Confidence interval
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- LOD:
-
Logistic organ dysfunction
- MDRD:
-
Modification of diet in renal disease
- MV:
-
Mechanical ventilation
- OR:
-
Odds ratio
- RI:
-
Doppler-based renal resistive index
- ROC curve:
-
Receiver-operating characteristic curve
- RRT:
-
Renal replacement therapy
- SQP:
-
Semi-quantitative perfusion
References
Prowle JR, Liu Y-L, Licari E, Bagshaw SM, Egi M, Haase M et al (2011) Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care 15(4):R172
Bagshaw SM (2008) Short- and long-term survival after acute kidney injury. Nephrol Dial Transplant 23(7):2126–2128
Metnitz PGH, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K et al (2002) Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 30(9):2051–2058
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG et al (2007) Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11(2):R31
Waikar SS, Bonventre JV (2009) Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20(3):672–679
Kellum JA, Lameire N (2013) KDIGO AKI guideline work group. diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 17(1):204
Darmon M, Schortgen F, Vargas F, Liazydi A, Schlemmer B, Brun-Buisson C et al (2011) Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med 37(1):68–676
Lerolle N, Guérot E, Faisy C, Bornstain C, Diehl J-L, Fagon J-Y (2006) Renal failure in septic shock: predictive value of Doppler-based renal arterial resistive index. Intensive Care Med 32(10):1553–1559
Schnell D, Deruddre S, Harrois A, Pottecher J, Cosson C, Adoui N et al (2012) Renal resistive index better predicts the occurrence of acute kidney injury than cystatin C. Shock 38(6):592–597
Platt JF, Rubin JM, Ellis JH (1991) Acute renal failure: possible role of duplex Doppler US in distinction between acute prerenal failure and acute tubular necrosis. Radiology 179(2):419–423
Schnell D, Reynaud M, Venot M, Le Maho AL, Dinic M, Baulieu M et al (2014) Resistive Index or color-Doppler semi-quantitative evaluation of renal perfusion by inexperienced physicians: results of a pilot study. Minerva Anestesiol 80(12):1273–1281
Dewitte A, Coquin J, Meyssignac B, Joannès-Boyau O, Fleureau C, Roze H et al (2012) Doppler resistive index to reflect regulation of renal vascular tone during sepsis and acute kidney injury. Crit Care 16(5):R165
Schnell D, Camous L, Guyomarc’h S, Duranteau J, Canet E, Gery P et al (2013) Renal perfusion assessment by renal Doppler during fluid challenge in sepsis. Crit Care Med 41(5):1214–1220
Ninet S, Schnell D, Dewitte A, Zeni F, Meziani F, Darmon M (2015) Doppler-based renal resistive index for prediction of renal dysfunction reversibility: a systematic review and meta-analysis. J Crit Care 30(3):629–635
Schnell D, Darmon M (2012) Renal Doppler to assess renal perfusion in the critically ill: a reappraisal. Intensive Care Med 38(11):1751–1760
Lerolle N (2012) Please don’t call me RI anymore; I may not be the one you think I am! Crit Care 16(6):174
Dewitte A, Joannès-Boyau O, Sidobre C, Fleureau C, Bats M-L, Derache P et al (2015) Kinetic eGFR and Novel AKI Biomarkers to predict renal recovery. Clin J Am Soc Nephrol 10(11):1900–1910
Kellum JA, Lameire N (2013) KDIGO AKI guideline work group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 17(1):204
Perinel S, Vincent F, Lautrette A, Dellamonica J, Mariat C, Zeni F et al (2015) Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit Care Med 43(8):269–275
Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM et al (2017) Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol 13(4):241–257
Le Gall JR, Klar J, Lemeshow S, Saulnier F, Alberti C, Artigas A et al (1996) The logistic organ dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU scoring group. JAMA 276(10):802–810
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13(10):818–829
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D et al (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med 29(4):530–538
Hajian-Tilaki K (2014) Sample size estimation in diagnostic test studies of biomedical informatics. J Biomed Inform 48:193–204
Vittinghoff E, McCulloch CE (2007) Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 165(6):710–718
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C et al (2011) pROC: an open-source package for R and S + to analyze and compare ROC curves. BMC Bioinform 17(12):77
Fawcett T (2006) An introduction to ROC analysis. Pattern Recogn Lett 27(8):861–874
Perkins NJ, Schisterman EF (2006) The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 163(7):670–675
Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G (2015) Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol 26(9):2231–2238
Izumi M, Sugiura T, Nakamura H, Nagatoya K, Imai E, Hori M (2000) Differential diagnosis of prerenal azotemia from acute tubular necrosis and prediction of recovery by Doppler ultrasound. Am J Kidney Dis 35(4):713–719
Stevens PE, Gwyther SJ, Hanson ME, Boultbee JE, Kox WJ, Phillips ME (1990) Noninvasive monitoring of renal blood flow characteristics during acute renal failure in man. Intensive Care Med 16(3):153–158
Tublin ME, Tessler FN, Murphy ME (1999) Correlation between renal vascular resistance, pulse pressure, and the resistive index in isolated perfused rabbit kidneys. Radiology 213(1):258–264
Bude RO, Rubin JM (1999) Relationship between the resistive index and vascular compliance and resistance. Radiology 211(2):411–417
Murphy ME, Tublin ME (2000) Understanding the Doppler RI: impact of renal arterial distensibility on the RI in a hydronephrotic ex vivo rabbit kidney model. J Ultrasound Med 19(5):303–314
Darmon M, Schortgen F, Leon R, Moutereau S, Mayaux J, Di Marco F et al (2009) Impact of mild hypoxemia on renal function and renal resistive index during mechanical ventilation. Intensive Care Med 35(6):1031–1038
Naesens M, Heylen L, Lerut E, Claes K, De Wever L, Claus F et al (2013) Intrarenal resistive index after renal transplantation. N Engl J Med 369(19):1797–1806
Cook NR (2007) Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 115(7):928–935
de Grooth H-J, Parienti J-J, Schetz M (2018) AKI biomarkers are poor discriminants for subsequent need for renal replacement therapy, but do not disqualify them yet. Intensive Care Med 44(7):1156–1158
Funding
This study was supported by Saint-Etienne University Hospital
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
M.D. declares having received administrative support from his former institution (Saint-Etienne University Hospital) to conduct this study and having received research support from Astute Medical unrelated to the current study. The other authors declare no conflict of interest related to this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Darmon, M., Bourmaud, A., Reynaud, M. et al. Performance of Doppler-based resistive index and semi-quantitative renal perfusion in predicting persistent AKI: results of a prospective multicenter study. Intensive Care Med 44, 1904–1913 (2018). https://doi.org/10.1007/s00134-018-5386-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5386-3