Abstract
Summary
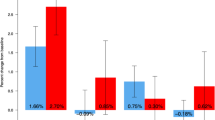
We evaluate 38 elderly women who had received long-term denosumab treatment after stopping the drug. Taking into account the gain during treatment and the loss after stopping treatment, they lost 35.5% of the total gain in the spine, 44.6% of the total gain in the femoral neck, and 103.3% in the total hip.
Introduction
Denosumab (DMAb) is a soluble inhibitor of the receptor activator of nuclear factor-kappaB ligand (RANKL) and, therefore, does not incorporate into the bone matrix. Consistently, DMAb discontinuation is associated with reversal of the effects attained with treatment.
Purpose
The aim of this study is to assess changes in BMD after a year of discontinuation of DMAb in a group of postmenopausal women treated with DMAb for 7 or 10 years. Secondly, is to evaluate the occurrence of fragility fractures.
Methods
Women who had participated in the FREEDOM study and its extension were invited to participate in this follow-up study. BMD at LS and hip and spine X-rays were obtained. Results were compared to the last value obtained while in treatment to assess changes after discontinuation.
Results
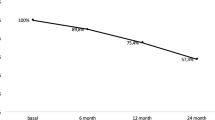
Thirty-eight women, mean age: 81 ± 3.4 years completed study procedures; none had received bisphosphonates after stopping DMAb. Mean gap time between DMAb last dose and the follow-up visit was 17 months (range 16–20 months). Bone mineral density (BMD) decreased significantly in all regions: − 8.1% in LS, − 6% in FN, and − 8.4% in TH. Five (5/38, 13.15%) patients had a fragility fracture, one suffered a wrist fracture, and four experienced vertebral fractures. Three patients suffered one vertebral fracture and one of them had two vertebral fractures. Laboratory results showed the following mean values: CTX = 996 ± 307 pg/ml (normal values 550 ± 226 pg/ml); osteocalcin = 55.2 ± 18.6 ng/ml (normal value 42 ng/ml); and 25 OH vitamin D = 23.7 ± 6.9 ng/ml.
Conclusion
Our results describe the rapid bone loss occurring after cessation of denosumab treatment. Further studies are needed to assess if patients have a higher risk of fracture after stopping DMAb and if so, which patients have the highest risk, and assess the role of transitioning to bisphosphonates in the long term.
Similar content being viewed by others
References
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, ZoogHB AM, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C, Trial F (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Langdahl BL, Teglbjærg CS, Ho PR, Chapurlat R, Czerwinski E, Kendler DL, Reginster JY et al (2015) A 24-month study evaluating the efficacy and safety of denosumab for the treatment of men with low bone mineral density: results from the ADAMO trial. J Clin Endocrinol Metab 100(4):1335–1342. https://doi.org/10.1210/jc.2014-4079
Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L et al (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377(9768):813–822. https://doi.org/10.1016/S0140-6736(10)62344-6
Stopeck A., Martin M., Ritchie D., Body J.J., Paterson A., Viniegra M., et al. (2010) Effect of denosumab versus zoledronic acid treatment in patients with breast cancer and bone metastases: results from the extended blinded treatment phase. Cancer Res 2010: P6-14-01–P6-14-01. https://doi.org/10.1016/j.ejca.2015.09.011
Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, Borenstein J et al (2012 Jan) Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23(1):317–326. https://doi.org/10.1007/s00198-011-1780-1
Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwiński E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, Roux C, Malouf J, Bradley MN, Daizadeh NS, Wang A, Dakin P, Pannacciulli N, Dempster DW, Papapoulos S (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5(7):513–523. https://doi.org/10.1016/S2213-8587(17)30138-9
Jamal SA, Ljunggren O, Stehman-Breen C, Cummings SR, McClung MR, Goemaere S, Ebeling PR, Franek E, Yang YC, Egbuna OI, Boonen S, Miller PD (2011) Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 26(8):1829–1835. https://doi.org/10.1002/jbmr.403
Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y, San Martin J, AMG 162 Bone Loss Study Group (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43:222–229
Bone HG, BologneseMA YCK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96:972–980
Brown JP, Roux C, Törring O, Ho PR, Beck Jensen JE, Gilchrist N, Recknor C, Austin M, Wang A, Grauer A, Wagman RB (2013) Discontinuation of denosumab and associated fracture incidence: analysis from the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial. J Bone Miner Res 28:746–752
Brown JP, Ferrari S, Gilchrist N, Beck Jensen JE, Pannacciulli N, Recknor C, et al. (2016) Discontinuation of denosumab and associated fracture incidence: analysis from FREEDOM and its extension. J Bone Miner Res 31 (Suppl 1):Available at https://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=51d4e88b-f79d- 47e2-a15b-134f0c57b52e. Accessed September 27, 2016
McClung MR (2016) Cancel the denosumab holiday. Osteoporos Int 27(5):1677–1682
Anastasilakis AD, Makras P (2016) Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporos Int 27(5):1929–1930
Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D, Lamy O (2016) Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int 27(5):1923–1925
Popp AW, Zysset PK, Lippuner K (2016) Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos Int 27(5):1917–1921
Polyzos SA, Terpos E (2016) Clinical vertebral fractures following denosumab discontinuation. Endocrine 54(1):271–272
Lamy O, Gonzalez-Rodriguez E, Stoll D, Hans D, Aubry-Rozier B (2016) Severe rebound-associated vertebral fractures after denosumab discontinuation: nine clinical cases report. J Clin Endocrinol Metab
Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O (2017) Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. https://doi.org/10.1002/jbmr.3110
McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM (2011) Observations following discontinuation of long-term denosumab therapy. J Bone Miner Res 26(8):1829–1835. https://doi.org/10.1002/jbmr.403
Anastasilakis AD, Yavropoulou MP, Makras P et al (2017) Increased osteoclastogenesis in patients with vertebral fractures following discontinuation of denosumab treatment. Eur J Endocrinol 176(6):677–683
Acknowledgements
We acknowledge Amgen’s support in data sharing and review of this manuscript.
We thank Susana Carballo for editorial assistance on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
María Belén Zanchetta, Juan Boailchuk, Fabio Massari, Fernando Silveira, Cesar Bogado, and José R. Zanchetta declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zanchetta, M.B., Boailchuk, J., Massari, F. et al. Significant bone loss after stopping long-term denosumab treatment: a post FREEDOM study. Osteoporos Int 29, 41–47 (2018). https://doi.org/10.1007/s00198-017-4242-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4242-6