Abstract:
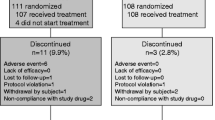
A number of drugs are now available for the treatment of established osteoporosis and have been shown to significantly increase bone mineral density (BMD). There are, however, few comparative treatment studies and, furthermore, adverse events remain a problem with some of the newer agents, particularly in the elderly, in everyday clinical practice. We report a 12 month, open labeled, randomized controlled, prospective treatment study in 140 postmenopausal women with established vertebral osteoporosis, comparing the effect of continuous alendronate, cyclical alendronate and cyclical etidronate with calcitriol in terms of gain in BMD, reduction in bone turnover markers and adverse event profile. The mean percentage increases in BMD at 12 months, at the spine and hip respectively, were: continuous alendronate 5.7%, 2.6%; cyclical alendronate 4.1%, 1.6%; cyclical etidronate 4.9%, 2.0% (p<0.01) and calcitriol 2.0%, 0.4% (NS). In comparison with calcitriol, the mean changes in BMD at the spine and hip respectively were greater in the other groups; continuous alendronate: 3.7% (95% CI 1.4 to 8.3), 2.2% (95% CI 0.7 to 4.0); cyclical alendronate: 2.1% (95% CI 1.2 to 6.4), 1.2% (95% CI −0.3 to 3.0); cyclical etidronate: 2.9% (95% CI 1.9 to 6.5), 1.6% (95% CI 0.9 to 3.1)). The reduction in bone turnover markers was between 26% and 32% in the alendronate and etidronate groups (p<0.01), with a trend toward greater reduction in the continuous alendronate group. Eight patients discontinued the study: 6 in the continuous alendronate group, 1 in the cyclical alendronate group and 1 in the calcitriol group. Two patients in the cyclical etidronate group were unable to tolerate the Cacit component, but continued on substituting Cacit with Calcichew. In summary, 12 months of treatment with continuous alendronate, cyclical alendronate and cyclical etidronate are effective in terms of the gain in BMD at the anteroposterior spine and total hip in a comparable treatment population. These treatments are more effective than calcitriol and were generally well tolerated. Continuous alendronate showed a trend toward a larger gain in BMD and greater suppression of bone turnover markers than the other treatment groups, but had a higher incidence of adverse events, particularly within the older subgroup. Cyclical alendronate offers a lower adverse event profile and appears to be effective in comparison with continuous treatment, and may possibly be an alternative in the elderly. However, further studies are necessary, but more importantly with fracture end-points.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 6 April 1999 / Accepted: 8 June 2000
Rights and permissions
About this article
Cite this article
Sahota, O., Fowler, I., Blackwell, P. et al. A Comparison of Continuous Alendronate, Cyclical Alendronate and Cyclical Etidronate with Calcitriol in the Treatment of Postmenopausal Vertebral Osteoporosis: A Randomized Controlled Trial . Osteoporos Int 11, 959–966 (2000). https://doi.org/10.1007/s001980070035
Issue Date:
DOI: https://doi.org/10.1007/s001980070035