Abstract
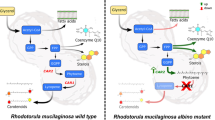
In this study, relationship between translucent property of yeast cell wall and occurrence of cyanobacteria inside the yeast vacuole was examined. Microscopic observations on fruit yeast Candida tropicalis showed occurrence of bacterium-like bodies inside the yeast vacuole. Appearance of vacuoles as distinct cavities indicated the perfect harvesting of light by the yeast’s cell wall. Transmission electron microscopy observation showed electron-dense outer and electron-lucent inner layers in yeast cell wall. Cyanobacteria-specific 16S rRNA gene was amplified from total DNA of yeast. Cultivation of yeast in distilled water led to excision of intracellular bacteria which grew on cyanobacteria-specific medium. Examination of wet mount and Gram-stained preparations of excised bacteria showed typical bead-like trichomes. Amplification of cyanobacteria-specific genes, 16S rRNA, cnfR and dxcf, confirmed bacterial identity as Leptolyngbya boryana. These results showed that translucent cell wall of yeast has been engineered through evolution for receiving light for vital activities of cyanobacteria.





Similar content being viewed by others
References
Adams DG (2002) Cyanobacteria in symbiosis with hornworts and liverworts. In: Rai AN, Bergman B, Rasmussen U (eds) Cyanobacteria in symbiosis. Springer, Netherlands, pp 117–135
Adams DG, Bergman B, Nierzwicki-Bauer SA, Duggan PS, Rai AN, Schüßler A (2013) Cyanobacterial-plant symbioses. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F (eds) The prokaryotes: prokaryotic biology and symbiotic associations. Springer, Berlin, pp 359–400
Ahmadjian V (1993) The lichen symbiosis. Wiley, New York
Almon H, Böhme H (1980) Components and activity of the photosynthetic electron transport system of intact heterocysts isolated from the blue–green alga Nostoc muscorum. Biochim Biophys Acta 592:113–120
Bae G, Choi G (2008) Decoding of light signals by plant phytochromes and their interacting proteins. Annu Rev Plant Biol 59:281–311
Bandyopadhyay A, Elvitigala T, Liberton M, Pakrasi HB (2013) Variations in the rhythms of respiration and nitrogen fixation in members of the unicellular diazotrophic cyanobacterial genus Cyanothece. Plant Physiol 161:1334–1346
Barghoorn ES (1971) The oldest fossils. Sci Am 224:30–43
Berman-Frank I, Lundgren P, Falkowski P (2003) Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res Microbiol 154:157–164
Bielecki S, Galas E (1991) Microbial β-glucanases different from cellulases. Crit Rev Biotechnol 10:275–304
Blumenstein A et al (2005) The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr Biol 15:1833–1838
Briggs WR, Spudich JL (2005) Handbook of photosensory receptors. Wiley, New York
Carpenter EJ, Janson S (2000) Intracellular cyanobacterial symbionts in the marine diatom Climacodium frauenfeldianum (Bacillariophyceae). Eur J Phycol 36:540–544
Cheng H-R, Jiang N (2006) Extremely rapid extraction of DNA from bacteria and yeasts. Biotechnol Lett 28:55–59
Davis SJ, Vener AV, Vierstra RD (1999) Bacteriophytochromes: phytochrome-like photoreceptors from nonphotosynthetic eubacteria. Science 286:2517–2520
Dutta P, Khatua M, Dutta J, Prasad R (2003) Use of Chitosan-DMAc/LiCl gel as drug carriers. Int J Chem Sci 1:93
Edgar RS et al (2012) Peroxiredoxins are conserved markers of circadian rhythms. Nature 485:459
Edwards H, Holder J, Wynn-Williams D (1998) Comparative FT-Raman spectroscopy of Xanthoria lichen-substratum systems from temperate and Antarctic habitats. Soil Biol Biochem 30:1947–1953
Fay P (1992) Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev 56:340–373
Giraud E et al (2002) Bacteriophytochrome controls photosystem synthesis in anoxygenic bacteria. Nature 417:202
Gualtieri P (2001) Chapter 10 Rhodopsin-like-proteins: light detection pigments in Leptolyngbya, Euglena, Ochromonas, Pelvetia. In: Häder D-P, Breure AM (eds) Comprehensive series in photosciences. Elsevier, pp 281–295
Gupta V, Natarajan C, Kumar K, Prasanna R (2011) Identification and characterization of endoglucanases for fungicidal activity in Anabaena laxa (Cyanobacteria). J Appl Phycol 23:73–81
Haberle RM et al (1993) Atmospheric effects on the utility of solar power on mars. In: Resources of near-earth space, p 845
Hasegawa M et al (2018) Molecular characterization of DXCF cyanobacteriochromes from the cyanobacterium Acaryochloris marina identifies a blue-light power sensor. J Biol Chem 293:1713–1727
Herrera-Estrella A, Horwitz BA (2007) Looking through the eyes of fungi: molecular genetics of photoreception. Mol Microbiol 64:5–15
Heydari S, Siavoshi F, Ebrahimi H, Sarrafnejad A, Sharifi AH (2019) Excision of endosymbiotic bacteria from yeast under aging and starvation stresses. Infect Genet Evol 78:104141
Hien NH, Fleet GH (1983) Separation and characterization of six (1 leads to 3)-beta-glucanases from Saccharomyces cerevisiae. J bacteriol 156:1204–1213
Hoffmeister M, Martin W (2003) Interspecific evolution: microbial symbiosis, endosymbiosis and gene transfer. Environ Microbiol 5:641–649
Hughes J et al (1997) A prokaryotic phytochrome. Nature 386:663
Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57:395–418
Jiang Z, Swem LR, Rushing BG, Devanathan S, Tollin G, Bauer CE (1999) Bacterial photoreceptor with similarity to photoactive yellow protein and plant phytochromes. Science 285:406–409
Karniol B, Wagner JR, Walker JM, Vierstra RD (2005) Phylogenetic analysis of the phytochrome superfamily reveals distinct microbial subfamilies of photoreceptors. Biochem J 392:103–116
Kehoe DM, Grossman AR (1996) Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science 273:1409–1412
Kluge M (2002) A fungus eats a cyanobacterium: the story of the Geosiphon pyriformis endocyanosis. Biol Environ J (PRIA) JSTOR 102:11–14.
Kluge M, Mollenhauer D, Wolf E, Schüßler A (2002) The Nostoc–Geosiphon endocytobiosis. In: Cyanobacteria in symbiosis. Springer, pp 19–30
Kneip C, Lockhart P, Voß C, Maier U-G (2007) Nitrogen fixation in eukaryotes—new models for symbiosis. BMC Evol Biol 7:55
Lipke PN, Ovalle R (1998) Cell wall architecture in yeast: new structure and new challenges. J Bacteriol 180:3735–3740
Lu W, Evans EH, McColl SM, Saunders VA (1997) Identification of cyanobacteria by polymorphisms of PCR-amplified ribosomal DNA spacer region. FEMS Microbiol Lett 153:141–149
Lucock M et al (2018) Photobiology of vitamins. Nutr Rev 76:512–525
Manchester LC et al (2015) Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res 59:403–419
Meeks JC (1998) Symbiosis between nitrogen-fixing cyanobacteria and plants. J Biosci 48:266–276
Meunier PC, Colon-Lopez MS, Sherman LA (1997) Temporal changes in state transitions and photosystem organization in the unicellular, diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. Plant Physiol 115:991–1000
Moran NA, Wernegreen JJ (2000) Lifestyle evolution in symbiotic bacteria: insights from genomics. Trends Ecol Evol 15:321–326
Mullineaux CW (2001) How do cyanobacteria sense and respond to light? Mol Microbiol 41:965–971
Murray PA, Zinder SH (1985) Nutritional requirements of Methanosarcina sp. strain TM-1. Appl Environ Microbiol 50:49–55
Nichols HW (1973) Growth media-freshwater. In: JR S (ed) Handbook of phycological methods: culture methods and growth measurements. Cambridge University Press, Cambridge, pp 7–24
Nicol S (1991) Life after death for empty shells. New Sci 129:46–48
Nübel U, Garcia-Pichel F, Muyzer G (1997) PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microbiol 63:3327–3332
Pillai C, Paul W, Sharma CP (2009) Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci 34:641–678
Pisciotta JM, Zou Y, Baskakov IV (2010) Light-dependent electrogenic activity of cyanobacteria. PLoS ONE 5:e10821
Rasmussen B, Fletcher IR, Brocks JJ, Kilburn MR (2008) Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455:1101
Rodriguez-Romero J, Hedtke M, Kastner C, Müller S, Fischer R (2010) Fungi, hidden in soil or up in the air: light makes a difference. Annu Rev Microbiol 64:585–610
Schmitz O, Katayama M, Williams SB, Kondo T, Golden SS (2000) CikA, a bacteriophytochrome that resets the cyanobacterial circadian clock. Science 289:765–768
Schnepf E, Schlegel I, Hepperle D (2002) Petalomonas sphagnophila (Euglenophyta) and its endocytobiotic cyanobacteria: a unique form of symbiosis. Phycologia 41:153–157
Siavoshi F et al (2019) Sequestration inside the yeast vacuole may enhance Helicobacter pylori survival against stressful condition. Infect Genet Evol 69:127–133
Strong C, Bullard J, Burford M, McTainsh G (2013) Response of cyanobacterial soil crusts to moisture and nutrient availability. CATENA 109:195–202
Tanaka H, Phaff HJ (1965) Enzymatic hydrolysis of yeast cell walls I. Isolation of wall-decomposing organisms and separation and purification of lytic enzymes. J Bacteriol 89:1570–1580
Thacker R, Starnes S (2003) Host specificity of the symbiotic cyanobacterium Oscillatoria spongeliae in marine sponges, Dysidea spp. Mar Biol 142:643–648
Tsujimoto R, Kamiya N, Fujita Y (2014) Transcriptional regulators ChlR and CnfR are essential for diazotrophic growth in nonheterocystous cyanobacteria. Proc Natl Acad Sci 111:6762–6767
Usher KM, Sutton DC, Toze S, Kuo J, Fromont J (2005) Inter-generational transmission of microbial symbionts in the marine sponge Chondrilla australiensis (Demospongiae). Mar Freshw Res 56:125–131
Vukusic P, Sambles JR (2003) Photonic structures in biology. Nature 424:852
Yoshida T, Sakamoto T (2009) Water-stress induced trehalose accumulation and control of trehalase in the cyanobacterium Nostoc punctiforme IAM M-15. J Gen Appl Microbiol 55:135–145
Yoshihara S, Suzuki F, Fujita H, Geng XX, Ikeuchi M (2000) Novel putative photoreceptor and regulatory genes required for the positive phototactic movement of the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 41:1299–1304
Zavrel M, Majer O, Kuchler K, Rupp S (2012) Transcription factor Efg1 shows a haploinsufficiency phenotype in modulating the cell wall architecture and immunogenicity of Candida albicans. Eukaryot Cell 11:129–140
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Yusuf Akhter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
203_2020_1835_MOESM1_ESM.mp4
Supplementary Video 1. Light microscopy of C. tropicalis. Video record shows fast-moving bacterium-like bodies inside the vacuole of yeast cells. Original magnification × 1250 (MP4 16878 kb)
203_2020_1835_MOESM2_ESM.avi
Supplementary Video 2. Live/Dead staining of C. tropicalis. Video record shows several live and actively moving bacterium-like bodies inside the yeast vacuole. Original magnification × 1000 (AVI 30237 kb)
Rights and permissions
About this article
Cite this article
Ebrahimi, H., Siavoshi, F., Heydari, S. et al. Yeast engineered translucent cell wall to provide its endosymbiont cyanobacteria with light. Arch Microbiol 202, 1317–1325 (2020). https://doi.org/10.1007/s00203-020-01835-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-01835-w