Abstract
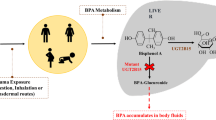
Bisphenol A (BPA) and bisphenol F (BPF) are widely used to manufacture plastics and epoxy resins. Both compounds have been shown to be present in the environment and are food contaminants, with, as a result, a low but chronic exposure of humans. However, the fate and possible bioactivation of these compounds at the level of human cell lines was not completely elucidated yet. In this study, we investigated the ability of human cells (intestinal cell line: LS174T, hepatoma cell line: HepG2, and renal cell line: ACHN) to biotransform BPA and BPF, and focused on the cytotoxicity and genotoxicity of these two bisphenols, through the use of a novel and efficient genotoxic assay based on the detection of histone H2AX phosphorylation. BPA and BPF were extensively metabolized in HepG2 and LS174T cell lines, with stronger biotransformation capabilities in intestinal cells than observed in liver cells. Both cell lines produced the glucuronide as well as the sulfate conjugates of BPA. Conversely, the ACHN cell line was found to be devoid of any metabolic capabilities for the two examined bisphenols. Cytotoxicity was tested for BPA, BPF, as well as one metabolite of BPF produced in vivo in rat, namely dihydroxybenzophenone (DHB). In the three cell lines used, we observed similar ranges of toxicity, with DHB being weakly cytotoxic, BPF exhibiting an intermediary cytotoxicity, and BPA being the most cytotoxic compound tested. BPA and DHB were not found to be genotoxic, whatever the cell line examined. BPF was clearly genotoxic in HepG2 cells. These results demonstrate that some human cell lines extensively metabolize bisphenols and establish the genotoxic potential of bisphenol F.




Similar content being viewed by others
References
Anderson D, Yu TW, McGregor DB (1998) Comet assay responses as indicators of carcinogen exposure. Mutagenesis 13:539–555
Atkinson A, Roy D (1995) In vivo DNA adduct formation by bisphenol A. Environ Mol Mutagen 26:60–66
Audebert M, Riu A, Jacques C, Hillenweck A, Jamin EL, Zalko D, Cravedi JP (2010) Use of the gammaH2AX assay for assessing the genotoxicity of polycyclic aromatic hydrocarbons in human cell lines. Toxicol Lett 199:182–192
Barnes L, Dumas M, Juan M, Noblesse E, Tesniere A, Schnebert S, Guillot B, Moles JP (2010) GammaH2AX, an accurate marker that analyzes UV genotoxic effects on human keratinocytes and on human skin. Photochem Photobiol 86:933–941
Bondesson M, Jonsson J, Pongratz I, Olea N, Cravedi JP, Zalko D, Hakansson H, Halldin K, Di Lorenzo D, Behl C, Manthey D, Balaguer P, Demeneix B, Fini JB, Laudet V, Gustafsson JA (2009) A CASCADE of effects of bisphenol A. Reprod Toxicol 28:563–567
Braniste V, Jouault A, Gaultier E, Polizzi A, Buisson-Brenac C, Leveque M, Martin PG, Theodorou V, Fioramonti J, Houdeau E (2010) Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc Natl Acad Sci USA 107:448–453
Bursztyka J, Perdu E, Pettersson K, Pongratz I, Fernandez-Cabrera M, Olea N, Debrauwer L, Zalko D, Cravedi JP (2008) Biotransformation of genistein and bisphenol A in cell lines used for screening endocrine disruptors. Toxicol In Vitro 22:1595–1604
Cabaton N, Chagnon MC, Lhuguenot JC, Cravedi JP, Zalko D (2006) Disposition and metabolic profiling of bisphenol F in pregnant and nonpregnant rats. J Agric Food Chem 54:10307–10314
Cabaton N, Zalko D, Rathahao E, Canlet C, Delous G, Chagnon MC, Cravedi JP, Perdu E (2008) Biotransformation of bisphenol F by human and rat liver subcellular fractions. Toxicol In Vitro 22:1697–1704
Cabaton N, Dumont C, Severin I, Perdu E, Zalko D, Cherkaoui-Malki M, Chagnon MC (2009) Genotoxic and endocrine activities of bis(hydroxyphenyl)methane (bisphenol F) and its derivatives in the HepG2 cell line. Toxicology 255:15–24
Collins AR, Oscoz AA, Brunborg G, Gaivao I, Giovannelli L, Kruszewski M, Smith CC, Stetina R (2008) The comet assay: topical issues. Mutagenesis 23:143–151
Fenech M (2009) A lifetime passion for micronucleus cytome assays–reflections from Down Under. Mutat Res 681:111–117
Hanioka N, Naito T, Narimatsu S (2008) Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere 74:33–36
Hanioka N, Oka H, Nagaoka K, Ikushiro S, Narimatsu S (2011) Effect of UDP-glucuronosyltransferase 2B15 polymorphism on bisphenol A glucuronidation. Arch Toxicol. doi:10.1007/s00204-011-0690-5
Ibuki Y, Tani Y, Toyooka T (2008) UVB-exposed chlorinated bisphenol A generates phosphorylated histone H2AX in human skin cells. Chem Res Toxicol 21:1770–1776
Ismail IH, Wadhra TI, Hammarsten O (2007) An optimized method for detecting gamma-H2AX in blood cells reveals a significant interindividual variation in the gamma-H2AX response among humans. Nucleic Acids Res 35:e36
Iso T, Watanabe T, Iwamoto T, Shimamoto A, Furuichi Y (2006) DNA damage caused by bisphenol A and estradiol through estrogenic activity. Biol Pharm Bull 29:206–210
Ivett JL, Brown BM, Rodgers C, Anderson BE, Resnick MA, Zeiger E (1989) Chromosomal aberrations and sister chromatid exchange tests in Chinese hamster ovary cells in vitro. IV. Results with 15 chemicals. Environ Mol Mutagen 14:165–187
Iwanari M, Nakajima M, Kizu R, Hayakawa K, Yokoi T (2002) Induction of CYP1A1, CYP1A2, and CYP1B1 mRNAs by nitropolycyclic aromatic hydrocarbons in various human tissue-derived cells: chemical-, cytochrome P450 isoform-, and cell-specific differences. Arch Toxicol 76:287–298
Izzotti A, Kanitz S, D’Agostini F, Camoirano A, De Flora S (2009) Formation of adducts by bisphenol A, an endocrine disruptor, in DNA in vitro and in liver and mammary tissue of mice. Mutat Res 679:28–32
Izzotti A, Longobardi M, Cartiglia C, D’Agostini F, Kanitz S, De Flora S (2010) Pharmacological modulation of genome and proteome alterations in mice treated with the endocrine disruptor bisphenol A. Curr Cancer Drug Targets 10:147–154
Jacques C, Perdu E, Dorio C, Bacqueville D, Mavon A, Zalko D (2010a) Percutaneous absorption and metabolism of [14C]-ethoxycoumarin in a pig ear skin model. Toxicol In Vitro 24:1426–1434
Jacques C, Perdu E, Duplan H, Jamin EL, Canlet C, Debrauwer L, Cravedi JP, Mavon A, Zalko D (2010b) Disposition and biotransformation of (14)C-Benzo(a)pyrene in a pig ear skin model: ex vivo and in vitro approaches. Toxicol Lett 199:22–33
Kirkland D, Pfuhler S, Tweats D, Aardema M, Corvi R, Darroudi F, Elhajouji A, Glatt H, Hastwell P, Hayashi M, Kasper P, Kirchner S, Lynch A, Marzin D, Maurici D, Meunier JR, Muller L, Nohynek G, Parry J, Parry E, Thybaud V, Tice R, van Benthem J, Vanparys P, White P (2007) How to reduce false positive results when undertaking in vitro genotoxicity testing and thus avoid unnecessary follow-up animal tests: report of an ECVAM workshop. Mutat Res 628:31–55
Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S (2005) Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci 84:249–259
Kurebayashi H, Okudaira K, Ohno Y (2010) Species difference of metabolic clearance of bisphenol A using cryopreserved hepatocytes from rats, monkeys and humans. Toxicol Lett 198:210–215
Lee M, Kwon J, Chung MK (2003) Enhanced prediction of potential rodent carcinogenicity by utilizing comet assay and apoptotic assay in combination. Mutat Res 541:9–19
Leepipatpiboon N, Sae-Khow O, Jayanta S (2005) Simultaneous determination of bisphenol-A-diglycidyl ether, bisphenol-F-diglycidyl ether, and their derivatives in oil-in-water and aqueous-based canned foods by high-performance liquid chromatography with fluorescence detection. J Chromatogr A 1073:331–339
Leopardi P, Cordelli E, Villani P, Cremona TP, Conti L, De Luca G, Crebelli R (2010) Assessment of in vivo genotoxicity of the rodent carcinogen furan: evaluation of DNA damage and induction of micronuclei in mouse splenocytes. Mutagenesis 25:57–62
Matsuzaki K, Harada A, Takeiri A, Tanaka K, Mishima M (2010) Whole cell-ELISA to measure the gammaH2AX response of six aneugens and eight DNA-damaging chemicals. Mutat Res 700:71–79
Mazur CS, Kenneke JF, Hess-Wilson JK, Lipscomb JC (2010) Differences between human and rat intestinal and hepatic bisphenol-A glucuronidation and the influence of alamethicin on in vitro kinetic measurements. Drug Metab Dispos 38:2232–2238
Muller L, Sofuni T (2000) Appropriate levels of cytotoxicity for genotoxicity tests using mammalian cells in vitro. Environ Mol Mutagen 35:202–205
Naik P, Vijayalaxmi KK (2009) Cytogenetic evaluation for genotoxicity of bisphenol-A in bone marrow cells of Swiss albino mice. Mutat Res 676:106–112
Naspinski C, Gu X, Zhou GD, Mertens-Talcott SU, Donnelly KC, Tian Y (2008) Pregnane X receptor protects HepG2 cells from BaP-induced DNA damage. Toxicol Sci 104:67–73
Nerin C, Philo MR, Salafranca J, Castle L (2002) Determination of bisphenol-type contaminants from food packaging materials in aqueous foods by solid-phase microextraction-high-performance liquid chromatography. J Chromatogr A 963:375–380
O’Brien PJ, Irwin W, Diaz D, Howard-Cofield E, Krejsa CM, Slaughter MR, Gao B, Kaludercic N, Angeline A, Bernardi P, Brain P, Hougham C (2006) High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch Toxicol 80:580–604
Perez P, Pulgar R, Olea-Serrano F, Villalobos M, Rivas A, Metzler M, Pedraza V, Olea N (1998) The estrogenicity of bisphenol A-related diphenylalkanes with various substituents at the central carbon and the hydroxy groups. Environ Health Perspect 106:167–174
Pritchett JJ, Kuester RK, Sipes IG (2002) Metabolism of bisphenol a in primary cultured hepatocytes from mice, rats, and humans. Drug Metab Dispos 30:1180–1185
Schweikl H, Schmalz G, Rackebrandt K (1998) The mutagenic activity of unpolymerized resin monomers in Salmonella typhimurium and V79 cells. Mutat Res 415:119–130
Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM (2010) Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res 704:152–159
Tayama S, Nakagawa Y, Tayama K (2008) Genotoxic effects of environmental estrogen-like compounds in CHO-K1 cells. Mutat Res 649:114–125
Trouiller B, Reliene R, Westbrook A, Solaimani P, Schiestl RH (2009) Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res 69:8784–8789
Tsuchiya Y, Nakajima M, Itoh S, Iwanari M, Yokoi T (2003) Expression of aryl hydrocarbon receptor repressor in normal human tissues and inducibility by polycyclic aromatic hydrocarbons in human tumor-derived cell lines. Toxicol Sci 72:253–259
Tsutsui T, Tamura Y, Yagi E, Hasegawa K, Takahashi M, Maizumi N, Yamaguchi F, Barrett JC (1998) Bisphenol-A induces cellular transformation, aneuploidy and DNA adduct formation in cultured Syrian hamster embryo cells. Int J Cancer 75:290–294
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177
Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G (2010) Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 118:1055–1070
Watters GP, Smart DJ, Harvey JS, Austin CA (2009) H2AX phosphorylation as a genotoxicity endpoint. Mutat Res 679:50–58
Yoshikawa T, Kashino G, Ono K, Watanabe M (2009) Phosphorylated H2AX foci in tumor cells have no correlation with their radiation sensitivities. J Radiat Res (Tokyo) 50:151–160
Zalko D, Soto AM, Dolo L, Dorio C, Rathahao E, Debrauwer L, Faure R, Cravedi JP (2003a) Biotransformations of bisphenol A in a mammalian model: answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ Health Perspect 111:309–319
Zalko D, Jaeg JP, Perdu-Durand E, Dolo L, Cravedi JP (2003b) Metabolic pathways of bisphenol A in precision-cut liver and intestine slices from CD1 mice. Toxicol Lett 144:S150–S151
Zhou C, Li Z, Diao H, Yu Y, Zhu W, Dai Y, Chen FF, Yang J (2006) DNA damage evaluated by gammaH2AX foci formation by a selective group of chemical/physical stressors. Mutat Res 604:8–18
Acknowledgments
LS174T cell line was a generous gift from Dr. J. Pouyssegur. This work was supported by the “Agence Nationale pour la Recherche” (ANR KISMET-2008-CESA-008-01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Audebert, M., Dolo, L., Perdu, E. et al. Use of the γH2AX assay for assessing the genotoxicity of bisphenol A and bisphenol F in human cell lines. Arch Toxicol 85, 1463–1473 (2011). https://doi.org/10.1007/s00204-011-0721-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-011-0721-2