Abstract
Tissue plasminogen activator (t-PA) has a short therapeutic time window for administration (3 h) and carries a risk of promoting intracerebral hemorrhage. The aim of the present study was to investigate a therapeutic time window and frequency of hemorrhagic region by treatment with Stachybotrys microspora triprenyl phenol-7 (SMTP-7). Thrombotic occlusion was induced by transfer of acetic acid-induced thrombus at the right common carotid artery into the brain of mice. Infarction area, neurological score, edema percentage, and regional cerebral blood flow (CBF) were determined as the index of the efficacy of SMTP-7. In order to evaluate the mechanism of SMTP-7, plasmin activities and the expressions of interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), and IL-6 mRNA were examined. SMTP-7 (0.1, 1, 10 mg/kg) dose dependently reduced infarction area, neurological score, and edema percentage. Additionally, its therapeutic time window was longer than that of t-PA, a high-molecular-weight compound. In addition, little hemorrhagic region was induced by treatment with SMTP-7. SMTP-7 showed plasmin activity in vivo and caused a decreased CBF to recover. Furthermore, the expressions of inflammatory cytokine mRNA (IL-1β, TNF-α, IL-6) were increased by t-PA treatment 3 h after ischemia but were not induced by SMTP-7 treatment. These results indicate that SMTP-7 shows potential thrombolytic and anti-inflammatory effects as well as a wide therapeutic time window and little hemorrhagic region compared with that of t-PA. Therefore, this novel low-molecular-weight compound may represent a novel approach for the treatment of cerebral infarction.
Similar content being viewed by others
Introduction
The triprenyl phenol staplabin (Shinohara et al. 1996) is the first low-molecular-weight compound that enhances both plasminogen–fibrin binding and the activation of plasminogen by relaxing the plasminogen conformation (Takayasu et al. 1997). Conformational modulation of plasminogen is thus an attractive means to regulate the localized plasminogen/plasmin system. Stachybotrys microspora IFO 30018 produces a variety of staplabin analogs (SMTPs) (Koyama et al. 1997; Hasumi et al. 1998), some of which are found to be several times as active as staplabin4. In particular, Stachybotrys microspora triprenyl phenol-7 (SMTP-7; Orniplabin, MW 869.1, Fig. 1) is five to ten times more potent than staplabin in enhancing plasminogen–fibrin binding, urokinase-catalyzed activation of plasminogen, and urokinase and plasminogen-mediated fibrin degradation (Hu et al. 2000).
Ischemic brain injury and subsequent cell death lead to long-term disabilities, such as paralysis, speech, and memory deficits and possibly death. Stroke is clearly a serious issue. There is limited satisfaction with drug therapy in this area because thrombolytic therapy can unfortunately only be used in a relatively low percentage of patients (The National Institute of Neurological Disorders, and Stroke rt-PA Stroke Study Group 1995; Wardlaw et al. 2003). Tissue plasminogen activator (t-PA), a high-molecular-weight compound, is the only approved therapy for acute stages in stroke. However, t-PA has a short therapeutic time window for administration (3 h) and carries a risk of promoting intracerebral hemorrhage (The National Institute of Neurological Disorders, and Stroke rt-PA Stroke Study Group 1997). Therefore, a method is needed to reduce t-PA-induced hemorrhage and extend the therapeutic time window for safe and effective reperfusion to limit cerebral damage. This is an important medical need. Several studies suggest that combination therapy with neuroprotective drugs may extend the t-PA treatment time window in embolic stroke (Okubo et al. 2007; Zhang et al. 2004; Murata et al. 2008). A large number of drugs have been developed but failed to expand the therapeutic time window and protect against reperfusion cerebral damage with single-agent administration.
Ischemic hypoxic brain injury often causes irreversible brain damage. The cascade of events leading to neuronal injury and death in ischemia includes the release of cytokines and free radicals, and induction of inflammation, apoptosis, and excitotoxicity (Kuroda and Siesjo 1997). When a tissue is subjected to ischemia and reperfusion, pro-inflammatory cytokines produced by inflammatory cells can trigger the adhesion and migration of circulating neutrophils to endothelial cells and the generation of reactive oxygen species (ROS) that enhance neutrophil infiltration and result in ischemic injury (Liou et al. 2003). Our goal is to develop an effective agent for treating cerebral infarction even by single-agent administration. We consider this to be an appropriate goal for helping many patients with embolic cerebral infarction. To achieve this goal, it is necessary to identify a novel compound that has dual activities of thrombolytic activity and an anti-inflammatory effect. Therefore, it is important that the mechanism of this drug is not limited to one factor but involves many mechanisms, most of which may be interrelated.
The aim of the present study was to investigate a therapeutic time window and frequency of hemorrhagic region by the treatment with SMTP-7. As a result, the present study demonstrated, for the first time, that SMTP-7 has a wide therapeutic time window and little hemorrhagic region in an embolic cerebral infarction model through its thrombolytic activity and anti-inflammatory effects.
Materials and methods
Materials
Sample buffer for fibrin zymography consisted of 125 mM of Tris-HCl, pH 6.8, 4% (w/v) sodium dodecyl sulfate (SDS), 0.04% (w/v) bromophenol blue, and 20% (w/v) sucrose. Acetylsalicylic acid (ASA) and 2,3,5-triphenyltetrazolium chloride (TTC) were purchased from Wako Pure Chemical (Osaka, Japan). Isoflurane (Escain®) was purchased from Mylan (Tokyo, Japan). SuperScript VILO cDNA Synthesis Kit, SYBR Green reagent system and TRIzol were purchased from Invitrogen (Carlsbad, CA, USA). The t-PA (Activacin®) was purchased from Kyowa Hakko Kirin (Tokyo, Japan).
Animals
Male ddY mice weighing 35–45 g (Saitama Jikken Co., Ltd., Saitama) were used. All experiments were conducted under the regulations of the Committee of Animal Care and Welfare of Showa University. Mice were maintained in an air-conditioned animal room at 20 ± 2°C, with 50 ± 20% relative humidity and a 12-hr light–dark cycle (lights on from 0800 to 2000 hours). The mice received a standard laboratory diet; in addition, water was provided ad libitum.
Embolic infarction model
The embolic infarction model was induced as described previously (Hashimoto et al. 2010). The mice were anesthetized with 5% isoflurane, and anesthesia was maintained with 1.5% isoflurane. The right common carotid artery (RCCA) was exposed carefully from the surrounding tissue. The RCCA was temporarily clamped using an aneurysm clip, and acetic acid was applied to generate a thrombus. After 10 min, the clip was removed, and the thrombus was transferred to the brain for embolization by the bloodstream. To evaluate the effect of surgical preparation, the same surgically operated mice without application of acetic acid were served as the sham-operated group. The control group was administered vehicle. Body temperature was maintained at 37°C during surgery with a heating lamp.
Evaluation of neurological scores
Twenty four hours after ischemia, the neurological scores were determined by a modified method described by Longa et al. (1989): no deficit, 0; failure to extend right hind limb fully, 1; circling to the right, 2; failing to the left, 3; no spontaneous walking with a depressed level of consciousness, 4.
Lesion size and brain swelling
The animals were killed after the evaluation of neurological scores. The brains were cut into 2-mm coronal slices starting at 1 mm from the frontal pole. The first and second slices were incubated with 2% TTC in saline for 30 min at 37°C. The TTC-unstained white area of the whole brain and the whole brain of each posterior face of the slice were measured by Image J software (version 1.40, NIH, Bethesda, MD, USA) and were numerically integrated across the thickness of the slice to obtain an estimate of the infarct volume and the whole brain volume, respectively. The volumes from all slices were summed to calculate the total infarct volume and were expressed as the percentage of the whole brain volume \( \left[ {{\hbox{infarct volume of whole brain}} = \left( {{\hbox{infarct volume of whole brain}}/{\hbox{volume of whole brain}}} \right) \times {1}00} \right] \). The degree of edema was calculated as follows: \( \left[ {{\hbox{edema percent}} = \left( {{\hbox{volume of potischemic hemisphere }}--{\hbox{ volume of contralateral hemisphere}}} \right)/{\hbox{volume of contralateral hemisphere}} \times {1}00} \right] \).
Evaluation of hemorrhagic transformation
The multifocal cerebral hemorrhages on the macroscopic brains were visually determined by consensus (K.S., T. H.). The rate of hemorrhage was calculated as the number of hemorrhagic cases divided by the total number of cases in each group.
Treatment protocol
The mice with cerebral infarction were randomly assigned to either the vehicle-treated group, SMTP-7-treated group, or t-PA-treated group. SMTP-7 was intravenously infused at doses of 0.1, 1, and 10 mg/kg as a 10% bolus and the remainder was continuously infused at a 30-min interval using a syringe pump (KDS200P, MUROMACHI KIKAI, Tokyo, Japan) at 1 h after embolization (n = 6). In other experiments, 10 mg/kg of SMTP-7 and t-PA were infused at 1 or 3 h after the embolization described above(n = 6, with exception of three experiments of cerebral blood flow). Previously, we confirmed that SMTP-7 and t-PA dose dependently inhibited the infarct area and neurological score in acetic acid-induced embolic cerebral model of the Mongolian gerbil and significant differences were observed at 10 mg/kg (i.v.) (Hashimoto et al. 2010). Therefore, we selected at 10 mg/kg in the present study. 10 mg/kg of ASA was infused as a bolus at 3 h after embolization just before t-PA administration (n = 6).
Fibrinogen zymography
Each blood sample was withdrawn in 3.8% trisodium citrate solution [1/9 (v/v)] at 0, 1, and 3 h after SMTP-7 or t-PA administration. The sample was centrifuged (5,000 rpm; 15 min; 4°C) and the plasma was stored with a sample buffer at −80°C until use. Zymography was performed with 7.5% SDS-polyacrylamide gel containing 2 mg/mL of fibrinogen. In this technique, each plasma sample was diluted 300-fold with distilled water and mixed with the SDS-polyacrylamide gel electrophoresis sample buffer without a reducing agent. Then, the samples were subjected to electrophoretic analysis at 4°C. The gels were sequentially washed twice in 2.5% Triton X-100 and incubated for 48 h in 0.1 M glycine and 50 mM of Tris-HCl (pH 8.3) at 37°C, stained for 30 min in Coomassie Brilliant Blue (CBB) R-250, and destained in acetic acid–methanol–water (1:1:6). Fibrinolytic activity was identified as negative staining with CBB R-250 and expressed as percentage of the positive control activity.
Laser Doppler perfusion imaging
CBF was determined by laser Doppler flowmetry (MoorFLPI, Moor Instruments Ltd., UK). CBF was measured before embolic infarction (100% baseline), after embolic infarction, and at 0, 1, 3, and 24 h after injection of t-PA or SMTP-7 in accordance with the manufacturer's instructions. The laser Doppler signal of the scanning image was analyzed using the MoorFLPI software (version 2.1). This software enables analysis of perfusion value profiles within a region of interest in circular areas of the same size containing the same number of pixels. Then, CBF was expressed as percentage of the pre-ischemic value.
Real-time reverse transcription-PCR analysis
Total RNA was isolated from the right and left sides of the third brain slice using the TRIzol method in accordance with the manufacturer's instructions. cDNA was generated from the RNA samples (1 μg) using a SuperScript VILO cDNA synthesis kit. Target cDNA levels were quantified by real-time reverse transcription-PCR (RT-PCR) using the ABI PRISM 7000 Sequence Detector (Applied Biosystems, Foster City, CA, USA) utilizing the SYBR green reagent system. The forward primer for β-actin was 5′-CCTTCCTTCTTGGGTATGGAATC-3′ and the reverse primer was 5′-TGCTAGGAGCCAGAGCAGTAATC-3′. The primers of interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), and IL-6 were purchased from Qiagen (Tokyo, Japan; QuantiTect Primer Assays) for mouse IL-1β (QT01048355), TNFα (QT00104006), and IL-6 (QT00098875). The thermal profile for the real-time RT-PCR was carryover prevention at 50°C for 2 min and initial activation step at 95°C for 15 min followed by 40 cycles of denaturation at 94°C for 15 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. Using the standard curve for the target of interest, relative copy number values can be determined for any unknown sample. The relative amount of each factor transcript was normalized to the amount of β-actin transcript in the same cDNA.
Statistical analysis
All data are expressed as the mean ± SEM. The statistical significance of a non-parametric test (neurological score) was determined by Steel test. Analysis of hemorrhage was done using the Fischer's exact test. The other statistical significance was evaluated with the one-way ANOVA followed by Bonferroni testing. P < 0.05 was considered statistically significant.
Results
Dose dependence of the effect of SMTP-7
Figure 2 illustrates the dose dependence of the effect of SMTP-7 in an acetic acid-induced embolic cerebral infarction model. Infarction area, neurological score, and edema percentage in the control group were 11.9 ± 2.54, 3.16 ± 0.477, and 13.2 ± 1.76, respectively. These values were significantly different from those of the sham-operated group. On the other hand, SMTP-7 ameliorated the size of the infarction area in a dose-dependent manner. Inhibition rates were 58.8% at 1 mg/kg and 78.9% at 10 mg/kg. There were statistically significant differences between the control group and the SMTP-7-treated group at 1 mg/kg (P < 0.05) and 10 mg/kg (P < 0.01). The neurological score and edema percentage were also improved by treatment with SMTP-7 at 10 mg/kg. Inhibition rates were 47.6% on neurological score and 58.5% on edema percentage. Significant differences were observed at 10 mg/kg (neurological score, P < 0.05; edema percentage, P < 0.01).
Concentration dependency of SMTP-7 on infarction area, neurological score, and edema percentage in embolic infarction model. SMTP-7 was administered at 1 h after ischemia. The values were obtained at 24 h after ischemia. Data represent the mean ± SEM of six experiments. *P < 0.05, **P < 0.01, statistically significant difference from control group
Extension of therapeutic time window
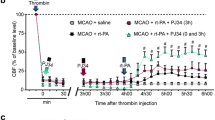
The next investigation compared the therapeutic time window for SMTP-7 with that for t-PA. Representative photographs of brain slices taken from mice subjected to SMTP-7 and t-PA treatments 3 h after ischemia are shown in Fig. 3a. In the t-PA-treated group at 3 h after ischemia, a clear infarction area was observed at the cerebral hemisphere affected by embolization. In contrast, the infarction area was not visible at 3 h after ischemia in the SMTP-7-treated group. The changes in infarction area, neurological score, and edema percentage over time are shown in Fig. 3b. Although t-PA ameliorated each variable by administration at 1 h after ischemia, its effect gradually decreased time-dependently. However, the effects of SMTP-7 on cerebral infarction area, neurological score, and edema percentage were maintained by administration at 3h after ischemia. There were significant differences between the SMTP-7-treated and control groups at all points. A combination of t-PA and ASA partially ameliorated the infarction area, neurological score, and edema percentage by treatment at 3 h after ischemia.
a Comparison of TTC-stained cerebral sections obtained from SMTP-7 and t-PA-treated embolic infarction models. The drugs were administered at 3 h after ischemia. Cerebral tissue was taken at 24 h after ischemia. b Comparative study of therapeutic time window in embolic infarction model. SMTP-7 and t-PA were administered at 1 or 3 h after ischemia. ASA was administered with t-PA at 3 h after ischemia. The values were obtained at 24 h after ischemia. Data represent the mean ± SEM of six experiments. *P < 0.05, **P < 0.01, statistically significant difference from control group
Hemorrhagic formation
Figure 4 illustrates the hemorrhagic formation. In the case of treatment with t-PA (10 mg/kg), the hemorrhagic formation was observed in a parietal region and the number of hemorrhage was 7/14. However, there was little hemorrhagic region by the treatment with 10 mg/kg SMTP-7 (1/13). There is a statistically significant difference between the SMTP-7- and t-PA-treated groups (P < 0.05).
a Comparison of the hemorrhagic formation in a parietal region obtained from SMTP-7 and t-PA-treated embolic infarction models. The brain was taken at 24 h after ischemia. b Comparison of the frequency of hemorrhagic formation obtained from SMTP-7 and t-PA-treated embolic infarction model. *P < 0.05, statistically significant difference between SMTP-7 and t-PA-treated groups
Fibrinolytic activity
Figure 5 shows the fibrinolytic activity in mice plasma after SMTP-7 and t-PA administrations. SMTP-7 caused a significant increase in fibrinolytic activity of about 3.5-fold at 1 and 3 h after administration (P < 0.01). On the other hand, t-PA exhibited a significantly greater increase in fibrinolytic activity at 0 h after administration (P < 0.01). However, the activity gradually decreased time-dependently. At 3 h after administration, the fibrinolytic activity of SMTP-7 was higher than that of t-PA.
Fibrinolytic activity of SMTP-7 and t-PA-treated embolic infarction models at 0, 1, and 3 h after drug administration. Drugs were administered at 1 h after ischemia. Fibrinolytic activity was identified as negative staining with CBB R-250 and expressed as a percentage of the positive control activity. Data represent the mean ± SEM of six experiments. *P < 0.05, **P < 0.01, statistically significant difference from control group
Change in CBF
After embolization, CBF of the ipsilateral hemisphere markedly fell to about 30% of the pre-ischemic value in all groups. In the control group, CBF of the ipsilateral hemisphere was maintained until 24 h later. However, CBF of the ipsilateral hemisphere after SMTP-7 administration gradually recovered time-dependently. At 24 h after SMTP-7 administration, CBF of the ipsilateral hemisphere recovered up to 76% of the pre-ischemic value. There were statistically significant differences between the post-ischemic value and at 24 h after SMTP-7 administration (P < 0.05). On the other hand, in the t-PA-treated group, CBF of the ipsilateral hemisphere quickly recovered at 1 h after t-PA administration. There were statistically significant differences between the post-ischemic value and at 1, 3, and 24 h after t-PA administration (P < 0.05) (Fig. 6a, b). Figure 6c illustrates the change in CBF of the ipsilateral hemisphere by t-PA administration at 1 or 3 h after ischemia. No significant difference in the recovery of CBF of the ipsilateral hemisphere was observed in a comparison between the two groups. Figure 6d illustrates the change in CBF of the ipsilateral hemisphere by SMTP-7 administration at 1 or 3 h after ischemia. No significant difference in the recovery of CBF of the ipsilateral hemisphere was also observed in a comparison between the two groups.
Change in CBF in embolic infarction model over time. a Pseudocolor images of cerebral tissue. Color bar indicates CBF. b Change in CBF over time. SMTP-7 and t-PA were administered at 1 h after ischemia. c Comparison of change in CBF over time. The t-PA was administered at 1 or 3 h after ischemia. d Comparison of change in CBF over time. SMTP-7 was administered at 1 or 3 h after ischemia. CBF was measured before embolic infarction (pre, 100% baseline), after embolic infarction (post), and at 0, 1, 3, and 24 h after administration. CBF was expressed as a percentage of the pre-ischemic value. Data represent the mean ± SEM of three experiments. *P < 0.05, **P < 0.01, statistically significant difference from each post value
Contribution of inflammation
Figure 7 expresses the real-time RT-PCR analysis for IL-1β, TNF-α, and IL-6. In the control group, the levels of the TNF-α (P < 0.01) and IL-6 (P < 0.05) mRNA expression were significantly higher in the ipsilateral hemisphere compared with the sham-operated group. In the case of treatment with t-PA, the expression levels of IL-1β, TNF-α, and IL-6 were not enhanced by administration at 1 h after ischemia. However, the administration of t-PA at 3 h after ischemia significantly enhanced the expressions of IL-1β, TNF-α, and IL-6 (P < 0.01). In contrast, the expression levels of IL-1β, TNF-α, and IL-6 were not increased by the treatment with SMTP-7 not only at 1 h but also at 3 h after ischemia. A combination of t-PA and ASA partially ameliorated inflammatory cytokine mRNA by treatment at 3 h after ischemia. No changes in these levels of mRNA expression were observed in the contralateral hemisphere.
Comparative study of mRNA expression of IL-1β, TNF-α, and IL-6. SMTP-7 and t-PA were administered at 1 or 3 h after ischemia. ASA was administered with t-PA at 3 h after ischemia. Samples were taken at 24 h after ischemia. Data represent the mean ± SEM of six experiments. *P < 0.05 and **P < 0.01 vs ipsilateral hemisphere of sham-operated group. # P < 0.05 and ## P < 0.01 vs ipsilateral hemisphere of control group
Discussion
We report here that SMTP-7 ameliorates embolic infarction and that its efficacy is greater than that of t-PA. This is the first low-molecular-weight compound with dual activities of thrombolytic and anti-inflammatory activity. We theorized that this compound overcomes the weakness of t-PA, namely, its narrow therapeutic time window. The risk of cerebral hemorrhage may limit the therapeutic time window of t-PA. In the present study, SMTP-7 showed an extended therapeutic time window (Fig. 3). Therefore, the problem of a narrow therapeutic time window may be resolved by SMTP-7 administration. On the other hand, t-PA carried hemorrhagic formation. However, little hemorrhagic region was induced by treatment with SMTP-7 (Fig. 4). Because plasminogen is activated on the surface of cells and the embolus, the effect of SMTP-7 is based on a physiological process. In other words, this compound enables the local amplification of plasmin at the sites at which generation is needed. This is one reason why SMTP-7 appears to cause less hemorrhage.
As shown in Fig. 5, we demonstrated that SMTP-7 had fibrinolytic activity in vivo. However, its activity is less than that of t-PA. In spite of lower fibrinolytic activity, the CBF of the ipsilateral hemisphere significantly recovered at 24 h after SMTP-7 administration (Fig. 6b). The discrepancy between fibrinolytic activity and recovery of CBF may be explained by different effector sites for the two drugs. SMTP-7 promotes conversion to plasmin by a conformational change of plasminogen with a plasminogen activator (Koyama et al. 1997). Because plasminogen is activated on the surface of cells and the embolus, this compound enabled local amplification of plasmin at the sites where it was needed. This is one reason why SMTP-7 caused full recovery of the CBF; at the same time, less hemorrhage appeared to occur. This feature of SMTP-7 is advantageous for the treatment of cerebral infarction. In addition, SMTP-7 is effective even when administered at 3 h after ischemia, although t-PA hardly had any effects. When t-PA was administered at 3 h after ischemia, reperfusion damage of cerebral tissue may occur because CBF recovers like that upon its administration at 1 h after ischemia (Fig. 6c). On the other hand, when SMTP-7 was administered at 3 h after ischemia, reperfusion damage of cerebral tissue did not occur.
Reperfusion of occluded vessels leads to the production of ROS. ROS can stimulate ischemic cells to secrete various inflammatory cytokines. IL-1β and TNF-α are cytokines that initiate the inflammatory response. IL-1β mRNA expression is increased at early reperfusion (1 h) and subsequently (6–24 h) (Davies et al. 1999). TNF-α is also upregulated in the brain after ischemia with a similar expression pattern to IL-1β (Murakami et al. 2005). IL-6 is thought to be a pro-inflammatory cytokine. The mRNA expression of these cytokines was enhanced by treatment with t-PA at 3 h after ischemia. In contrast, no significant increase in the mRNA expression of these inflammatory cytokines was observed by treatment with SMTP-7 at 3 h after ischemia (Fig. 7). In other words, reperfusion damage of cerebral tissue does not occur in the case of SMTP-7 treatment. This is also an important advantage over t-PA treatment. Combined administration with other drugs is unnecessary if SMTP-7 is used for the treatment of cerebral infarction. It is unclear from the present study why SMTP-7 suppresses inflammatory cytokine mRNA expression. Additional studies are required to clarify the precise mechanism involved.
As described above, upon treatment with t-PA at 3 h after ischemia, reperfusion damage was observed. At later times after ischemia, many kinds of gene induction occur (Koistinaho and Hokfelt 1997), some of which are also known to be involved in the brain inflammation that is a major factor in the progression of injury (Dirnagl et al. 1999; Barone and Feuerstein 1999). Therefore, we tested whether a combination of t-PA and ASA confers additional neuroprotection against reperfusion damage by treatment at 3 h after ischemia. The infarction area, neurological score, edema percentage, and inflammatory cytokine mRNA were partially ameliorated by this combination (Figs. 3b, 7). Therefore, we hypothesized that a few advantage was expected by combination therapy of t-PA and non-steroidal drug. Then, the novel drug having dual activity like a SMTP-7 is needed.
Many therapeutic agents show effectiveness in preclinical study using a small animal model but fail to exhibit positive effects in humans. As such, the stroke therapy academic industry roundtable meeting recommended that the evaluation of stroke recovery drugs should be conducted with a gyrencephalic species, namely, one that is similar to humans, for example, macaque monkeys (Stroke Therapy Academic Industry Roundtable 1999). We are also evaluating the effect of SMTP-7 using a thrombotic middle cerebral artery occlusion model in cynomolgus monkeys. We obtained positive effects nowadays. We will submit this observation in the future.
In summary, we demonstrated that SMTP-7 had sufficient efficacy, a wide therapeutic time window, and little hemorrhagic region compared with t-PA. This compound is the first low-molecular-weight compound with dual activities of thrombolytic and anti-inflammatory activity. The excellent therapeutic time window and little hemorrhagic region of SMTP-7 may have contributed to both activities. Therefore, SMTP-7 may represent a novel approach to the treatment of cerebral infarction.
References
Barone FC, Feuerstein GZ (1999) Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab 19:819–834
Davies CA, Loddick SA, Toulmond S, Stroemer RP, Hunt J, Rothwell NJ (1999) The progression and topographic distribution of interleukin-1β expression after permanent middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 19:87–98
Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischemic stroke: an integrated view. Trends Neurosci 22:391–397
Hashimoto T, Shibata K, Nobe K, Hasumi K, Honda K (2010) A novel embolic model of cerebral infarction and evaluation of SMTP-7, a novel fungal triprenyl phenol metabolite. J Pharmacol Sci (in press)
Hasumi K, Ohyama S, Kohyama T, Ohsaki Y, Takayasu R, Endo A (1998) Isolation of SMTP-3, 4, 5 and -6, novel analogs of staplabin, and theireffects on plasminogen activation and fibrinolysis. J Antibiot 51:1059–1068
Hu W, Ohyama S, Hasumi K (2000) Activation of fibrinolysis by SMTP-7 and -8, novel staplabin analogs with a pseudosymmetric structure. J Antibiot 53:241–247
Koistinaho J, Hokfelt T (1997) Altered gene expression in brain ischemia. NeuroReport 8:i–viii
Koyama T, Hasumi K, Hamanaka A, Endo A (1997) SMTP-1 and -2, novel analogs of staplabin produced by Stachybotrys microspore IFO30018. J Antibiot 50:172–174
Kuroda S, Siesjo BK (1997) Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin Neurosci 4:199–212
Liou KT, Shen YC, Chen CF, Tsao CM, Tsai SK (2003) Honokiol protects rat brain from focal cerebral ischemia-reperfusion injury by inhibiting neutrophil infiltration and reactive oxygen species production. Brain Res 992:159–166
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle artery occlusion without craniectomy in rat. Stroke 20:84–91
Murakami Y, Saito K, Hara A, Zhu Y, Sudo K, Niwa M, Fujii H, Wada H, Ishiguro H, Mori H, Seishima M (2005) Increases in tumor necrosis factor-α following transient global cerebral ischemia do not contribute to neuron death in mouse hippocampus. J Neurochem 93:1616–1622
Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH (2008) Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke 39:3372–3377
Okubo S, Igarashi H, Kanamatsu T, Hasegawa D, Orima H, Katayama Y (2007) FK-506 extended the therapeutic time window for thrombolysis without the risk of hemorrhagic transformation in embolic rat stroke model. Brain Res 1143:221–227
Shinohara C, Hasumi K, Hatsumi W, Endo A (1996) Staplabin, a novel fungal triprenyl phenol which stimulates the binding of plasminogen to fibrin and U937 cells. J Antibiot 49:961–966
Stroke Therapy Academic Industry Roundtable (1999) Recommendation for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30:2752–2758
Takayasu R, Hasumi K, Shinohara C, Endo A (1997) Enhancement of fibrin binding and activation of plasminogen by staplabin through induction of conformational change in plasminogen. FEBS Lett 418:58–62
The National Institute of Neurological Disorders, and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333:1581–1587
The National Institute of Neurological Disorders, and Stroke rt-PA Stroke Study Group (1997) Intracerebral haemorrhage after intravenous t-PA therapy for ischaemic stroke. Stroke 28:2109–2118
Wardlaw JM, del Zoppo G., Yamaguchi T, Berge E (2003) Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev (3):CD000213
Zhang W, Sato K, Hayashi T, Omori N, Nagano I, Kato S, Horiuti S, Abe K (2004) Extension of ischemic therapeutic time window by a free radical scavenger, edaravone, reperfused with tPA in rat brain. Neurol Res 26:342–348
Acknowledgments
This work was supported in part by a Showa University special grant-in-aid for innovative collaborative research project and a special research grant-in-aid for development of characteristic education from the Japanese Ministry of Education, Culture, Sports, Science and Technology. This work was also supported by the High-Tech Research Center Project for private universities, matching fund subsidy from MEXT (Ministry of Education, Culture, Sports, Science and Technology). SMTP-7 was generously donated by TMS Co., Ltd. (Tokyo, Japan).
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Shibata, K., Hashimoto, T., Nobe, K. et al. A novel finding of a low-molecular-weight compound, SMTP-7, having thrombolytic and anti-inflammatory effects in cerebral infarction of mice. Naunyn-Schmied Arch Pharmacol 382, 245–253 (2010). https://doi.org/10.1007/s00210-010-0542-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-010-0542-5