Abstract
Selenium nanoparticles (SeNPs) are well reported to exhibit pharmacological activities both in vitro and in vivo. However, literature is devoid of studies on the impact of SeNPs and/or metformin (M) against streptozotocin (STZ)-mediated oxidative brain injury and behavioral impairment. Consequently, to fill this gap, diabetes was induced in male Wistar rats by feeding with 10% fructose solution for 2 weeks, followed by a single dose intraperitoneal injection of STZ (40 mg/kg body weight [bwt]). After rats were confirmed diabetic, they were treated orally with 0.1 mg/kg bwt of SeNPs ± M (50 mg/kg bwt), and normal control (NC) received citrate buffer (2 mg/mL) for 5 weeks. In comparison with the diabetic control (DC), SeNPs, and/or M significantly (p < 0.05) lowered blood glucose levels, but increased insulin secretion and pancreatic β-cell function. An increase in locomotor and motor activities evidenced by improved spontaneous alternation, locomotor frequency, hinding, and increased mobility time were observed in treated groups. In addition, there was enhanced brain antioxidant status with a lower acetylcholinesterase (AChE) activity and oxidative-inflammatory stress biomarkers. A significant downregulation of caspase 3 and upregulation of parvalbumin and Nrf2 protein expressions was observed in treated groups. In some of the studied parameters, treated groups were statistically (p < 0.05) insignificant compared with the normal control (NC) group. Overall, co-treatment elicited more efficacy than that of the individual regimen.
Similar content being viewed by others
Introduction
Diabetes is a non-infectious metabolic disorder. It is characterized by hyperglycemia contingent on a shortage in secretion or resistance to insulin action and β-cell dysfunction (Adedara et al. 2019). Epidemiological studies revealed that the incidence of diabetes is on the rise around the world, with an estimation of approximately 387 million people. This figure is predicted to rise to 590 million by 2035 (Guariguata et al. 2014). This rise in the incidence of diabetes is of global concern, as it has been linked to immunocompromise that could exacerbate viral infections like COVID-19. Therefore, studies on mechanisms and new pharmacological agents with hypoglycemic potentials to enhance the well-being of people with diabetes are warranted.
Among the complications associated with diabetes are damage of vital organs functions, including the brain (Banks et al. 2012; Ascher-Svanum et al. 2015). Neurological disorders owing to diabetes have been confirmed in both clinical and experimental studies (Erbaş et al. 2016; Bădescu et al. 2016). Several studies have shown that diabetes-related alterations in brain function could aggravate neurological ailments, for instance, impaired cognitive function, depression, anxiety, and altered locomotor function (Kodl and Seaquist 2008; Patel and Udayabanu 2017). Earlier reports revealed a noticeably higher frequency in anxiety among diabetic subjects in comparison with their non-diabetic counterparts (Maia et al. 2014; Castellano-Guerrero et al. 2018). The vulnerability of the brain to diabetes can be ascribed to its high level of peroxidizable polyunsaturated fatty acids (PUFAs) constituent, which is the principal target of peroxidative attack under conditions of oxidative stress. Oxidative stress could occur in diabetes owing to increased production of free radicals, which prevail over the body’s inherent endogenous antioxidant systems (Patel and Udayabanu 2017). Altered pancreatic β-cells function and insulin resistance are the main features of diabetes (Kodl and Seaquist 2008; Adedara et al. 2019) and are triggered by oxidative stress owing to the continual high glucose level in the system (Asmat and Abad 2016; Ebokaiwe et al. 2019).
Metformin is the most commonly used oral regimen in diabetic treatment. The role of metformin in the treatment of neurodegenerative diseases apart from the known hypoglycemic effect has garnered attention in recent times (Markowicz-Piasecka et al. 2017). Results of several experimental and clinical studies indicate that metformin treatment improves cognitive function (Markowicz-Piasecka et al. 2017) and protect the brain against the oxidative imbalance imposed by diabetes (Correia et al. 2008). Alteration in selenium homeostasis is one of the various mechanisms proposed for the pathogenesis of diabetic complications. Earlier studies showed that there were increased risks of diabetes and its accompanying complications owing to deficient selenium levels in the brain (Ozkaya et al. 2009; Rayman and Stranges 2013). Thus, selenium plays a vital role in brain functions.
The emerging influence of nanotechnology as well as reported biological activities of nano-sized materials by ameliorating several metabolic disorders (Al-Quraishy et al. 2015; Abdulmalek and Balbaa 2019; Ebokaiwe et al. 2019, 2020a, b) prompted the current study on SeNPs. Also, recent developments in nanotechnological advancement have resulted in the nano-sizing of dietary trace elements with proven biological significance such as Se nanoparticles (SeNPs). These have garnered recognition in the management of metabolic disorders owing to their functions as composites of some enzymes and proteins in the biological system (Rayman and Stranges 2013; Abdulmalek and Balbaa 2019; Ebokaiwe et al. 2019, 2020a).
In this study, the basic features of diabetes were mimicked in rats using fructose to induce insulin resistance and a low dose of STZ to induce partial dysfunction in pancreatic β-cell per earlier protocol (Wilson and Islam 2012). Hence, this study is the first designed to assess the potential influence of SeNPs and/or Met treatment against diabetes-associated brain inflammatory/oxidative injury and behavioral impairments in rats.
Materials and methods
Chemicals
Selenium was procured from Thermo Fisher Acros Organics (Geel, Belgium). Anti-parvalbumin was purchased from Novus Biologicals (USA), anti-Nrf2 primary antibody from Abcam (USA), and anti-caspase 3 from Cell Signaling (USA), and β-actin antibody from Santa Cruz Biotechnology (USA). All chemicals/reagents were of analytical grade.
Production, lyophilization, and characterization of SeNPs
The nanoparticle was produced, lyophilized, and characterized by following the methods in our earlier reports (Ebokaiwe et al. 2019, 2020a).
Animals
Male adult Wistar rats weighing 165 g ± 5 g were selected for this study. Animals, obtained from the Animal House, Alex Ekwueme Federal University, Ndufu-Alike Ikwo, Nigeria, and kept under the 12 h light-dark cycle. Animals handling was with humane care and standard rat chow diet with water provided ad libitum.
Induction of T2D diabetes and experimental protocol
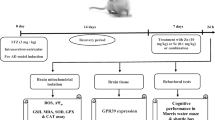
Following the protocol described by Wilson and Islam (2012), diabetes in animals was induced after a 1-week acclimatization period, by replacing the drinking water with 10% fructose solution ad libitum for 2 weeks to induce insulin resistance, while the normal control (NC) was given only drinking water. Intraperitoneal injection of STZ (40 mg/kg bwt) in citrate buffer (pH 4.5) at day 0 was administered to rats to induce partial pancreatic β-cell dysfunction. NC rats were administered the same quantity of citrate buffer. A week after induction of diabetes, the non-fasting blood glucose (NFBG) level was determined in the blood collected from the tail vein of all rats using an Accu-chek glucometer (Roche Diagnostics GmbH, Mannheim, Germany). Animals with NFBG level higher than 230 mg/dL were considered as diabetic and selected for the study.
As shown in Fig. 1 five groups of eight (8) rats each were maintained throughout the investigation period and administered as follows:
Group I, NC rats were orally administered water/citrate buffer (2 mL/mg bwt);
Group II: Diabetic control (DC) rats were orally administered with citrate buffer (2 mL/kg bwt);
Group III: Diabetic rats treated with metformin (M) orally at 50 mg/kg;
Group IV: Diabetic rats treated with SeNPs (0.1 mg/kg) orally;
Group V: Diabetic rats treated with SeNPs and M (0.1 and 50 mg/kg) orally.
Selected doses of SeNPs and M were chosen based on our earlier studies (Ebokaiwe et al. 2019, 2020a, b). Animals were monitored all through the 6-week intervention period for non-fasting blood glucose levels and body weight. Animals were sacrificed by cervical dislocation, 24 h after the last treatment, and the blood collected from the retro-orbital venous plexus. Serum samples were obtained by centrifuging blood cells for 10 min at 3000g. This was stored frozen and used later for the determination of insulin levels. Brain tissues were quickly excised, weighed, and some fixed in 10% neutral buffered formalin for immunohistochemistry. All others were frozen at − 80 °C until further biochemical estimations.
Antidiabetic evaluation
Rat Insulin ELISA kit-#90,010 (Crystal Chem, Zaandam, Netherlands) was used to quantify levels of rat insulin in serum. Homeostatic model assessment (HOMA-IR and HOMA-β) were evaluated with serum insulin levels and fasting blood glucose (FBG) concentrations taken at the end of the experiment using the following expression:
Evaluation of neurobehavioral indices
Tests were conducted after the last administration using the Y-maze, open field test (OFT), and tail suspension test (TST), following protocols from earlier studies (Mori et al. 2014; Ijomone et al. 2015, 2018). All behavioral tests were videotaped and later scored by two independent observers who were blinded to the experimental protocol. All apparatuses used for the tests were cleaned with 10% ethanol to remove possible bias due to smell left by previous animal.
Y-maze
The Y-maze was performed as previously described (Ijomone et al. 2015). This test evaluates short-term spatial memory as a measure of cognitive abilities using spontaneous alternating behaviors that is common in rats. Here, a three-armed Y-shaped maze was used. Rats were placed on a predetermined start arm and allowed to roam freely for 8 min. Arm entry (hind limbs completely in arm) was scored. Entering all 3 arms in the overlapping triplet sets is defined as spontaneous alternation. The percentage of spontaneous alternation was calculated as: [spontaneous alternation / (total number of arm entries – 2)] × 100.
Open field test
This assesses locomotor and exploratory activities in rats using protocols previously described (Ijomone et al. 2015, 2018). Here, an apparatus consisting of a box (72 × 72 × 36 cm) with the floor divided into 18 × 18 square units was used. Rats were placed in the center of the box and allowed to roam freely for 5 min. Locomotion frequency (number of crossings from one square to the other), rearing frequency (number of times the animals stood on their hind paws), rearing against the wall (number of times the animals stood on their hind paws against the wall), and hinding (calculated by adding the rearing frequency to rearing against the wall) are among the parameters scored.
Tail suspension test
This test assesses depressive or despair-like behavior in rats. The idea is based on the fact that rats will develop an immobile posture when exposed to unavoidable stress of being suspended by their tail (Ijomone et al. 2015, 2018). Here, rats were suspended individually by their tail from a retort stand with an adhesive tape for 6 min, and the amount of time each rat spent immobile was recorded.
Determination of antioxidant/oxidative stress, pro-inflammatory biomarkers, and acetylcholinesterase activity in rat brain
The supernatants from the brain samples were analyzed for antioxidant activities and oxidative stress biomarkers by following earlier protocols, superoxide dismutase (SOD) activity (Misra and Fridovich 1972), catalase (CAT) activity (Clairborne 1995), levels of reduced glutathione (GSH) (Jollow et al. 1974), glutathione-S-transferase (GST) activity (Habig et al. 1974), the activity of glutathione peroxidase (GSH-Px) (Rotruck et al. 1973), and levels of lipid peroxidation (LPO) (Garcia et al. 2005). To determine the level of pro-inflammation, the supernatants were analyzed for myeloperoxidase (MPO) activity (Eiserich et al. 1998) [31] and acetylcholinesterase (AChE) activity (Ellman et al. 1961).
Western blotting
Cell lysates from brain homogenates were prepared in the lysis buffer (50 mm Tris, pH 7.4 containing 0.15 M NaCl,10% glycerol (v/v), 1% NP-40 (v/v), 1 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM PMSF, 1 mM EDTA, 150 mM bestatin, 1 mM leupeptin, and 1 mM aprotinin) using a tissue:buffer ratio of 1:5. Protein concentration was estimated with BCA kit and equal amounts of protein were loaded on each lane and subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Mini Protean II System, Bio-Rad, Berkeley, CA) as described by Kim (2017) for anti-parvalbumin (#NB120–11427; Novus Biologicals, USA), anti-Nrf2 (#ab31163; Abcam, USA), and cleaved caspase 3 (#9664 Cell Signaling, USA).
Immunohistochemistry
A total of 5 μm thick sections of routine paraffin embedded brain tissues were used. Anti-Nrf2 (#ab31163; Abcam, MA, USA) proteins were analyzed following protocols from earlier studies (Ebokaiwe et al. 2019, 2020a, b).
Statistical analysis
Data were reported as mean ± SEM. Data comparisons were carried out using one-way ANOVA and subsequently by Newman-Keuls multiple comparison test. GraphPad Prism (version 5.03; GraphPad Software, La Jolla, CA, USA) was utilized in plotting charts and analyzing data. Statistical significance was set at p < 0.05.
Results
Blood glucose levels, body weight gain, organ weight, and survival rate
Blood glucose concentrations were significantly higher in all diabetic groups when compared with the normal group post-STZ injection. However, blood glucose levels were reduced in some groups of diabetic rats when treated with SeNPs and the standard of care drug metformin in comparison with untreated groups throughout the intervention period (Table 1) (day 7 [F4,30 = 59,432; p < 0.0001], day 14 [F4,30 = 13,613; p < 0.0001], day 21 [F4,30 = 13,408; p < 0.0001], day 28 [F4,30 = 16,294; p < 0.0001], day 35 [F4,30 = 18,447; p < 0.0001], day 42 [F4,30 = 13,656; p < 0.0001]). The gain in body weight was significantly different in rats in the DC group compared with treated groups and the NC [F4,30 = 417; p < 0.0001] (Table 2). It decreased after 2 weeks of treatment and continued to reduce steadily throughout the study period. Significant lower brain weight was observed in the DC group compared with that of the NC and treated groups at the end of the study period (F4,30 = 78; p < 0.0001) (Table 2). Also observed was a higher mortality rate in the DC group after day 14 than the NC and treated groups (Table 2).
Serum insulin concentration as well as HOMA-IR and HOMA-β scores in various animal groups at the end of the study period
Insulin concentration was lower in DC group when compared with the NC and treated groups (F4,30 = 366; p < 0.0001) (Table 3). A corresponding higher HOMA-IR in the serum of the DC was observed than that of the NC and treated groups, whereas, a lower level of HOMA-β was observed in the DC than that of the NC and the treated groups (Table 3).
SeNPs and/or M improved locomotor activities and exploratory profile in diabetic rats
Figure 2 shows the results of endpoint analyses of locomotor activities and exploratory profile in various groups during the 8 min trial in the novel environment. A significant reduction in total time immobile, total distance traveled, average speed, and spontaneous alternation (angle of an absolute turn and total body rotation) was observed in the DC group when compared with treated groups and NC.
Neurobehavioral indices of different experimental groups at the end of the treatment period. a Spontaneous alternation. b Immobility time. c Hinding. d Locomotion frequency. Data (n = 8) presented as mean ± SEM. ***p < 0.001 compared with NC; ##p < 0.001 compared with DC. NC (normal control), DC (diabetic control), D + M (diabetic + 50 mg/kg bwt metformin), D + SeNPs (diabetic + 0.1 mg/kg bwt SeNPs), D + SeNPs/M (diabetic + 0.1/50 mg/kg bwt SeNPs and metformin)
The evidence of improved locomotor activities and exploratory profile are shown in Fig. 2a; the Y-maze test revealed a significant decline in percentage of spontaneous alternation in DC than that of the NC and treated rats (F4,30 = 208; p < 0.0001). (Fig. 2b) The TST revealed a significant increase in immobility time in DC compared to the NC and treated groups (F4,30 = 35; p < 0.0001). Activities in the OFT (Fig. 2c and d) showed a significant decrease in both hinding and locomotion frequency in DC when compared with the NC and treated groups (hinding [F4,30 = 475; p < 0.0001], locomotion frequency [F4,30 = 149; p < 0.0001]). Co-administration of SeNPs and standard drug (M) showed greater efficacy in reversing the diabetes-induced neurobehavioral alterations in comparison with the individual treatment groups, i.e., locomotion frequency (DC vs NC [q = 32], DC vs D + M [q = 14], DC vs D + SeNPs [q = 14], DC vs D + SeNPs/M [q = 23]), spontaneous alternation (DC vs NC [q = 35], DC vs D + M [q = 21], DC vs D + SeNPs [q = 20], DC vs D + SeNPs/M [q = 35]), hinding (DC vs NC [q = 40], DC vs D + M [q = 19], DC vs D + SeNPs [q = 14], DC vs D + SeNPs/M [q = 52]), immobility time (DC vs NC [q = 15], DC vs D + M [q = 9], DC vs D + SeNPs [q = 8], DC vs D + SeNPs/M [q = 15]).
SeNPs and/or M improved antioxidant enzyme activities and GSH levels in the brain of diabetic rats
The antioxidant activities are shown in Fig. 3 a, SOD; b, CAT; c, GST; d, GSH-Px; and e, levels of GSH in the brain of NC and experimental rats. We observed a noticeable decline in the activities of SOD, CAT, and glutathione enzymes (GST and GSH-Px), as well as GSH levels, in the brain of DC rats when compared with the NC and treated groups (SOD [F4,30 = 97; p < 0.0001], CAT [F4,30 = 68; p < 0.0001], GST [F4,30 = 427; p < 0.0001], GSH-Px [F4,30 = 27; p < 0.0001], GSH [F4,30 = 84; p < 0.0001]. Administration of SeNPs and/or M significantly reversed the alterations in the brain antioxidant system to levels indistinguishable from the NC. These data indicate that treatment with SeNPs and/or M elicit their beneficial effects in reversing brain injury associated with diabetes by improving the antioxidant system.
Antioxidant parameters of different experimental groups at the end of the study period. a SOD. b CAT. c GST. d GSH. e GSH-Px. Data (n = 8) presented as mean ± SEM. ***p < 0.001 compared with NC; ##p < 0.001 compared with DC. NC (normal control), DC (diabetic control), D + M (diabetic + 50 mg/kg bwt metformin), D + SeNPs (diabetic + 0.1 mg/kg bwt SeNPs), D + SeNPs/M (diabetic + 0.1/50 mg/kg bwt SeNPs and metformin)
SeNPs and/or M lowers lipid peroxidation, pro-inflammatory biomarkers, and acetylcholinesterase activities in diabetic rats
The dynamics of the levels of lipid peroxidation, activities of myeloperoxidase, and acetylcholinesterase in the brain of NC and experimental groups of rats are presented in Fig. 4a, b, and c. The DC group showed a significant increase in the levels of LPO (which confirms oxidative stress), a significant increase in MPO, consistent with pro-inflammatory effects, and upsurge in acetylcholinesterase activity. These parameters were significantly decreased and indistinguishable from the NC group, following treatment with SeNPs and/or M (LPO [F4,30 = 41; p < 0.0001], MPO [F4,30 = 44; p < 0.0001], AChE [F4,30 = 1446; p < 0.0001]. This implies that intervention with SeNPs and/or M elicit their beneficial effects in reversing brain injury associated with diabetes through inhibition of oxidative stress/inflammation and a concomitant decrease in acetylcholinesterase activity.
Levels of lipid peroxidation, myeloperoxidase and acetylcholinesterase activities in different experimental groups at the end of the study period. a levels of LPO. b MPO activity. c AChE activity. Data (n = 8) presented as mean ± SEM. ***p < 0.001 compared with NC; ##p < 0.001 compared with DC. NC (normal control), DC (diabetic control), D + M (diabetic + 50 mg/kg bwt metformin), D + SeNPs (diabetic + 0.1 mg/kg bwt SeNPs), D + SeNPs/M (diabetic + 0.1/50 mg/kg bwt SeNPs and metformin)
SeNPs and/or M regulate Nrf2, caspase 3, and parvalbumin, expression in diabetic rats
Figure 5 represents the expression and immunoreactivity of Nrf2, Fig. 6, parvalbumin, and caspase 3 protein expression in the NC and experimental groups. The expression and immunoreactivity of Nrf2 protein was significantly lower in the brain of DC rats compared with that of the NC. Interestingly, treatment with SeNPs and/or M significantly restored these alterations relative to NC (F4,30 = 151; p < 0.0001) (Fig. 5). Parvalbumin (PV) expression decreased while caspase 3 expression increased following induction of diabetes. SeNPs and/or M groups showed significant attenuation of low PV and high caspase 3 expression in the brain (PV [F4,30 = 185; p < 0.0001], CAS 3 [F4,30 = 179; p < 0.0001] (Fig. 6). In all the analyzed proteins, SeNPs and M co-treatment demonstrated a better efficacy than that of their individual treatment. Nrf2 expression (DC vs NC [q = 26], DC vs D + M [q = 21], DC vs D + SeNPs [q = 25], DC vs D + SeNPs/M [q = 33]), CAS 3 (DC vs NC [q = 28], DC vs D + M [q = 20], DC vs D + SeNPs [q = 27], DC vs D + SeNPs/M [q = 36]), PV (DC vs NC [q = 28], DC vs D + M [q = 27], DC vs D + SeNPs [q = 27], DC vs D + SeNPs/M [q = 37]) (Fig. 7).
Nrf2 protein expression at the end of the study period. a Immunoreactivity. b Western blot expression. Data (n = 8) presented as mean ± SEM. ***p < 0.001 compared with NC; ##p < 0.001 compared with DC. NC (normal control), DC (diabetic control), D + M (diabetic + 50 mg/kg bwt metformin), D + SeNPs (diabetic + 0.1 mg/kg bwt SeNPs), D + SeNPs/M (diabetic + 0.1/50 mg/kg bwt SeNPs and metformin)
Cleaved caspase 3 and parvalbumin protein expression at the end of the study period. a Cleaved caspase 3 expression. b Parvalbumin expression. Data (n = 8) presented as mean ± SEM. ***p < 0.001 compared with NC; ##p < 0.001 compared with DC. NC (normal control), DC (diabetic control), D + M (diabetic + 50 mg/kg bwt metformin), D + SeNPs (diabetic + 0.1 mg/kg bwt SeNPs), D + SeNPs/M (diabetic + 0.1/50 mg/kg bwt SeNPs and metformin)
Discussion
The use of STZ to induce diabetes in vivo and in vitro to understand the complications associated with diabetes and the possible modulatory efficacy of known drugs and new compounds is of interest to researchers, following the global rise in diabetes conditions (Kamat et al. 2016). Neurotoxicity of STZ exposure involves altered glucose metabolism, insulin signaling, oxidative stress, and apoptosis (Kamat et al. 2016; Biswas et al. 2016). The two key pathological conditions of diabetes which are insulin resistance and partial pancreatic β-cell dysfunction was successfully exhibited in the experimental animals in this study. Although normal functioning of the brain requires high glucose demand, the brain cells cannot cope with persistent glucose uptake under diabetic conditions due to hyperglycemia—a trend referred to as glucose neurotoxicity (Tomlinson and Gardiner 2008; Bahniwal et al. 2017). Consequently, the prevention or attenuation of diabetic neurotoxicity is of vital interest to the clinician in order to improve the health of diabetic patients.
Previous studies demonstrated that low doses of selenium could exhibit antidiabetic and insulin-mimetic activities in animal models (Steinbrenner et al. 2011; Al-Quraishy et al. 2015; Abdulmalek and Balbaa 2019; Ebokaiwe et al. 2019, 2020a, b). However, clinical interventions using selenium as a drug are contradictory, as low doses acute exposure show efficacy whereas high doses and long-term usage worsen diabetes by increasing insulin resistance (Thomson 2004; Steinbrenner et al. 2011; Fontenelle et al. 2018). An earlier study by Steinbrenner (2013) reported that high doses of selenium contributed to the induction of insulin resistance as a result of its role in the metabolism of carbohydrates and lipids. In another study elsewhere, Wang et al. (2014) demonstrated that high doses of selenium (200 mg/kg) exacerbated hyperglycemia by promoting the expression of carboxykinase phosphoenolpyruvate and glucose 6-phosphatase enzymes involved in gluconeogenesis. The study by Jablonska et al. (2016) demonstrated that low doses of selenium improve homeostasis of glucose and the expression of genes related to glucose metabolism at different levels of regulation, linked to insulin signaling, glycolysis, and pyruvate metabolism.
NFBG levels are a vital parameter in comprehending the severity of type 2 diabetes (Group 1998). We measured the NFBG from day 7 of the intervention and fasting blood glucose (FBG) at the end of the intervention. From our results, SeNPs and/or M exhibited potent blood glucose-lowering activity in the treated rats, starting from the second week and throughout the intervention. Potential mechanisms that may account for the blood glucose-lowering activity of SeNPs and/or M include enhancement of insulin action (Hwang et al. 2007) and pancreatic β-cell stimulation (Al-Quraishy et al. 2015). Earlier study by Deeds and colleagues have established significant weight loss and mortality of rodents as accomplices of STZ toxicity and complications of hyperglycemia. The noticeable reduction in body weight and high mortality in the DC group in this study corroborates earlier reports (Deeds et al. 2011) as part of the toxicology impact of STZ. However, bodyweight gain and blood glucose were significantly improved to levels comparable with the NC after treatment with SeNPs and/or M indicating an improvement in metabolic condition and attenuation of tissue damage associated with hyperglycemia in the treated groups.
Maintaining glucose homeostasis is undoubtedly significant to lower the risk of micro or macro-vascular complications in diabetes condition (Chawla et al. 2016). Treatment with SeNPs and/or M enhanced insulin concentration in diabetic rats compared to DC. This activity is due to lower NFBG and FBG levels observed in the treated groups, as a result of enhanced glucose uptake by the peripheral tissues. Furthermore, SeNPs and/or M reduced HOMA-IR index (for insulin resistance) and also improved the HOMA-β (for β-cell function) score. This technique (HOMA) is utilized in the estimation of insulin resistance and β-cell function from fasting blood glucose levels and insulin concentration (Wallace et al. 2004). The reduction in the HOMA-IR scores by SeNPs and/or M treatment has further strengthened their potent antidiabetic activities.
Diabetes is accompanied by altered cognitive and motor functions, as evidenced by a significant decrease in spontaneous alternation, reduced locomotion frequency, increased immobility time, and decreased hinding and forelimb grip, reflecting altered short-term memory, depression, and impairment in the coordination between the nervous and muscular junctions (Sharma et al. 2010; Adedara et al. 2019). We observed a similar trend in this study; however, the observed tendency to reverse impaired neurobehavioral parameters in diabetic rats following treatment with SeNPs and/or M is a further confirmation of metabolic improvement in the treated rats.
Reactive oxygen species (ROS) generation, oxidative stress, and altered levels of inflammatory mediators have been strongly implicated in tissue damage due to hyperglycemia (Newsholme et al. 2016). Estimation of antioxidant defense along with LPO provide insight into the brain redox status (Valko et al. 2016) since membranes within the brain are known to be rich in peroxidizable fatty acids, thus they undergo peroxidation under oxidative insult (Shichiri 2014). The current study demonstrated the efficacy in the treatment of diabetic rats with SeNPs and/or M, evidenced in their conspicuous ability to attenuate LPO by decreasing MDA level in treated rats. Accordingly, SeNPs and/or M not only lowered the LPO level but also increased both first and second line of antioxidant defense mechanisms. The present investigation showed that DC rats exhibited heightened MPO activity in the brain samples, which indicates the induction of inflammatory response in diabetic neurotoxicity. Acetylcholinesterase hydrolyzes acetylcholine, an essential neurotransmitter in the regulation of motor function and locomotion (Day et al. 1991). The observed decrease in MPO and AChE activity by SeNPs and/or M treatment indicates amelioration of inflammation and consequently, improving cholinergic neurotransmission and restoring locomotor functions. Several reports have implicated Nrf2 as the main transcription factor of antioxidative stress (Zhang et al. 2018; Ebokaiwe et al. 2019, 2020a, b). Also, activation of Nrf2 expression decreases secondary brain damage and improves functional recovery after traumatic brain injury (Chandran et al. 2017; Zhang et al. 2018; Ebokaiwe et al. 2019). In this study, the observed significant reduction in Nrf2 expression in DC animals is an indication of oxidant stress. Administration of SeNPs and/or M restored Nrf2 expression to basal levels, which could be one of the mechanisms/pathways involved in attenuating diabetes mediated neurobehavioral dysfunction.
The antioxidant activity of selenoproteins in the CNS is well recognized, and deficiency of Se elicits brain injury (Fang et al. 2013). In addition to the role of Se as an essential component of the antioxidant system in the brain, studies have further demonstrated that Se can assuage oxidative stress in the brain through the regulation of Ca2+ channels, mitochondrial biogenesis, and apoptosis (Steinbrenner and Sies 2013; Dominiak et al. 2016).
Altered calcium homeostasis could lead to severe brain damage in diabetic conditions. The reduction in levels of calcium-binding protein-parvalbumin in the brain samples has been reported under diabetic conditions (Park and Koh 2017). Parvalbumin, a known calcium-buffering protein that is structurally similar to calmodulin (Cates et al. 2002; Koh 2012) has an affinity for Ca2+-binding domains, thus playing a vital function in the maintenance of Ca2+ homeostasis. Hence, this protein is an important Ca2+-buffering protein (Silver and Erecińska 1990; Lindholm et al. 2002). During our investigation, DC rats showed reduced expression of parvalbumin in the whole brain lysate. The observed enhancement in the expression of parvalbumin following SeNPs and/or M treatment corroborates their beneficial neuro-therapeutic effects by modulating calcium homeostasis via regulation of parvalbumin protein expression, hence, another possible pathway that is involved in SeNPs and/or M attenuating influence against diabetes-induced brain damage. Hyperglycemia, hypoxia, and ischemic conditions have been reported to enhance intracellular Ca2+ levels as well as apoptotic and necrotic cell death in the rat brain (Lindholm et al. 2002; Park and Koh 2017). In neurodegenerative diseases, cellular dysfunction and cell death are usually mediated by increased cytoplasmic Ca2+ concentrations (Park and Koh 2017). In the present study, caspase 3 protein-apoptotic biomarker was estimated. Elevated expression of caspase 3 in the brain of DC animals was significantly attenuated in the SeNPs and/or M treated groups, an indication of apoptosis regulation.
Conclusion
According to the findings from this study, the restorative impact of SeNPs and/or M against diabetes-induced alterations in neurobehavioral and biochemical/molecular indices is attributed to the enhancement of endogenous antioxidant systems, reduced lipid peroxidation, suppression of oxidative/inflammatory stress, acetylcholinesterase, and most importantly regulation of molecular markers of oxidant stress and tissue damage, Nrf2, caspase-3, and parvalbumin proteins.
References
Abdulmalek SA, Balbaa M (2019) Synergistic effect of nano-selenium and metformin on type 2 diabetic rat model: diabetic complications alleviation through insulin sensitivity, oxidative mediators and inflammatory markers. PLoS One 14(8):e0220779
Adedara IA, Fasina OB, Ayeni MF, Ajayi OM, Farombi EO (2019) Protocatechuic acid ameliorates neurobehavioral deficits via suppression of oxidative damage, inflammation, caspase-3 and acetylcholinesterase activities in diabetic rats. Food Chem Toxicol 125:170–181
Al-Quraishy S, Dkhil MA, Abdel-Moneim AE (2015) Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomedicine 10:6741–6756
Ascher-Svanum H, Chen YF, Hake A, Kahle-Wrobleski K, Schuster D, Kendall D (2015) Cognitive and functional decline in patients with mild Alzheimer dementia with or without comorbid diabetes. Clin Ther 37(6):1195–1205
Asmat UK, Abad KI (2016) Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm J 24(5):547–553
Bădescu SV, Tătaru CP, Kobylinska L, Georgescu EL, Zahiu DM, Zăgrean AM, Zăgrean L (2016) Effects of caffeine on locomotor activity in streptozotocin-induced diabetic rats. J Med Life 9:275–279
Bahniwal M, Little JP, Klegeris A (2017) High glucose enhances neurotoxicity and inflammatory cytokine secretion by stimulated human astrocytes. Curr Alzheimer Res 14(7):731–741
Banks WA, Owen JB, Erickson MA (2012) Insulin in the brain: there and back again. Pharmacol Ther 136(1):82–93
Biswas J, Goswami P, Gupta S, Joshi N, Nath C, Singh S (2016) Streptozotocin induced neurotoxicity involves Alzheimer’s related pathological markers: a study on N2A cells. Mol Neurobiol 53(5):2794–2806
Castellano-Guerrero AM, Guerrero R, Relimpio F, Losada F, Mangas MA, Pumar A, Martinez-Brocca MA (2018) Prevalence and predictors of depression and anxietyin adult patients with type 1 diabetes in tertiary care setting. Acta Diabetol 55:943–953
Cates MS, Teodoro ML, Phillips GN Jr (2002) Molecular mechanisms of calcium and magnesium binding to parvalbumin. Biophys J 82:1133–1146
Chandran R, Kim TS, Mehta L (2017) A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J Cereb Blood Flow Metab 6:1–9
Chawla A, Chawla R, Jaggi S (2016) Microvascular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab 20(4):546–551
Clairborne A (1995) Catalase activity. In: Greewald AR (ed) Handbook of Methods for Oxygen Radical Research. CRC Press, Boca Raton, pp 237–242
Correia S, Carvalho C, Santos MS, Proença T, Nunes E, Duarte A, Monteiro P, Seiça R, Oliveira CR, Moreira PI (2008) Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med Chem 4:358–364
Day J, Damsma G, Fibiger HC (1991) Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo micro dialysis study. Pharmacol Biochem Behav 38:723–729
Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, Eberhardt NL, Kudva YC (2011) Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim 45:131–140
Dominiak A, Wilkaniec A, Wroczyński P, Adamczyk A (2016) Selenium in the therapy of neurological diseases. Where is it going? Curr Neuropharmacol 14:282–299
Ebokaiwe AP, Ijomone OM, Griffin S, Ehiri RC, Obeten KE, Nwankwo JO, Ejike CECC, Keck CM (2019) Nanosized selenium and Loranthus micranthus leaves ameliorate streptozotocin-induced hepato-renal dysfunction in rats via enhancement of antioxidant system, regulation of caspase 3 and Nrf2 protein expression. Pharmanutrition 9:100150
Ebokaiwe AP, Obeten KE, Okori SO, David EE, Olusanya O, Chukwu CJ, Okoro N, Ehiri RC (2020a) Co-administration of selenium nanoparticles and metformin abrogate testicular oxidative injury by suppressing redox imbalance, augmenting sperm quality and Nrf2 protein expression in streptozotocin-induced diabetic rats. Biol Trace Elem Res. https://doi.org/10.1007/s12011-020-02082-2
Ebokaiwe AP, Osawe S, Griffinc S, Keckc CM, Olusanya O, Ehiri RC (2020b) Loranthus micranthus nanoparticles abates streptozotocin-instigated testicular dysfunction in Wistar rats: involvement of glucose metabolism enzymes, oxido-inflammatory stress, steroidogenic enzymes/protein and Nrf2 pathway. Andrologia. https://doi.org/10.1111/and.13749
Eiserich JP, Hristova M, Cross CE (1998) Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature 391:393–397
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Erbaş O, Oltulu F, Yılmaz M, Yavaşoğlu A, Taşkıran D (2016) Neuroprotective effects of chronic administration of levetiracetam in a rat model of diabetic neuropathy. Diabetes Res Clin Pract 114:106–116
Fang KM, Cheng FC, Huang YL, Chung SY, Jian ZY, Lin MC (2013) Trace element, antioxidant activity, and lipid peroxidation levels in brain cortex of gerbils after cerebral ischemic injury. Biol Trace Elem Res 152:66–74
Fontenelle LC, Feitosa MM, Morais JBS, Severo JS, de Freitas TEC, Beserra JB, Henriques GS, Marreiro DN (2018) The role of selenium in insulin resistance. Braz J Pharm Sci 54(1):e00139
Garcia YJ, Rodríguez-Malaver AJ, Peñaloza N (2005) Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J Neurosci Methods 144(1):127–135
Group UK (1998) Prospective diabetes study, effect of intensive bloodglucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352(9131):854–865
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103:137–149
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase. The first enzymaticstep in mercapturic acid formation. J Biol Chem 249:7130–7139
Hwang D, Seo S, Kim Y, Kim C, Shim S, Jee S, Lee S, Jang M, Kim M, Yim S, Sang-Koo L, Kang B, Jang I, Cho J (2007) Selenium acts as an insulin-like molecule for the down-regulation of diabetic symptoms via endoplasmic reticulum stress and insulin signalling proteins in diabetes-induced non-obese diabetic mice. J Biosci 32:723–735
Ijomone OM, Olaibi OK, Biose IJ, Mba C, Tete SA, Nwoha PU (2015) Chronic nicotine administration does not alter cognitive or mood associated behavioural parameters. Pathophysiology 22(1):57–63
Ijomone OM, Olatunji SY, Owolabi JO, Naicker T, Aschner M (2018) Nickel-induced neurodegeneration in the hippocampus, striatum and cortex; an ultrastructural insight, and the role of caspase-3 and α-synuclein. J Trace Elem Med Biol 50:16–23
Jablonska E, Reszka E, Gromadzinska J, Wieczorek E, Krol MB, Raimondi S, Socha K, Borawska MH, Wasowicz W (2016) The effect of selenium supplementation on glucose homeostasis and the expression of genes related to glucose metabolism. Nutrients 8:772
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzeneoxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Kamat PK, Kalani A, Rai S, Tota SK, Kumar A, Ahmad A (2016) Streptozotocin intracerebroventricular-induced neurotoxicity and brain insulin resistance: a therapeutic intervention for treatment of sporadic Alzheimer’s disease (sAD)-like pathology. Mol Neurobiol 53(7):4548–4562
Kim B (2017) Western blot techniques. Methods Mol Biol 1606:133–139
Kodl CT, Seaquist ER (2008) Cognitive dysfunction and diabetes mellitus. Endocr Rev 29:494–511
Koh PO (2012) Ischemic injury decreases parvalbumin expression in a middle cerebral artery occlusion animal model and glutamate-exposed HT22 cells. Neurosci Lett 14:17–21
Lindholm D, Mercer EA, Yu LY, Chen Y, Kukkonen J, Korhonen L, Arumäe U (2002) Neuronal apoptosis inhibitory protein: structural requirements for hippocalcin binding andeffects on survival of NGF-dependent sympathetic neurons. Biochim Biophys Acta 1600:138–147
Maia AC, Braga A, Ade P, Machado S, Nardi AE, Silva AC (2014) Psychiatric comorbidity in diabetes type 1: a cross-sectional observational study. Rev Assoc Med Bras 60:59–62
Markowicz-Piasecka M, Sikora J, Szydłowska A, Skupień A, Mikiciuk-Olasik E, Huttunen KM (2017) Metformin–a future therapy for neurodegenerative diseases. Pharm Res 34:2614–2627
Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Mori YCD, Li X, Yin J, Wang Z, Zhang Y, Mao C, Zhou H, Liu Z (2014) Huperzine a ameliorates cognitive deficits in streptozotocin-induced diabetic rats. Int J Mol Sci 15:7667–7683
Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt Jr PI (2016) Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J 473:4527–4550
Ozkaya M, Sahin M, Cakal EK, Bilge GF, Kilinc M (2009) Selenium levels in first-degree relatives of diabetic patients. Biol Trace Elem Res 128:144–151
Park DJ, Koh PO (2017) Diabetes aggravates decreases in hippocalcin and parvalbumin expression in focal cerebral ischemia. Neurosci Lett. https://doi.org/10.1016/j.neulet.10.039
Patel SS, Udayabanu M (2017) Effect of natural products on diabetes associated neurological disorders. Rev Neurosci 28:271–293
Rayman MP, Stranges S (2013) Epidemiology of selenium and type 2 diabetes: can we make sense of it? Free Radic Biol Med 65:1557–1564
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Sharma AN, Elased KM, Garrett TL, Lucot JB (2010) Neurobehavioral deficits in db/db diabetic mice. Physiol Behav 101:381–388
Shichiri M (2014) The role of lipid peroxidation in neurological disorders. J Clin Biochem Nutr 54:151–160
Silver A, Erecińska M (1990) Intracellular and extracellular changes of [Ca2+] in hypoxia and ischemia in rat brain in vivo. J Gen Physiol 95:837–866
Steinbrenner H (2013) Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radic Biol Med 65:1538–1547
Steinbrenner H, Sies H (2013) Selenium homeostasis and antioxidant selenoproteins in brain: implications for disorders in the central nervous system. Arch Biochem Biophys 536:152–157
Steinbrenner H, Speckmann B, Pinto A, Sies H (2011) High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr 48(1):40–45
Thomson CD (2004) Assessment of requirements for selenium andadequacy of selenium status: a review. Eur J Clin Nutr 58:391–402
Tomlinson DR, Gardiner NJ (2008) Glucose neurotoxicity. Nat Rev Neurosci 9(1):36–45
Valko M, Jomova K, Rhodes CJ, Kuča K, Musilek K (2016) Redox- and non- redoxmetal-induced formation of free radicals and their role in human disease. Arch Toxicol 90:1–37
Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27(6):1487–1495
Wang X, Zhang W, Chen H, Liao N, Wang Z, Zhang X (2014) High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS. Toxicol Lett 224(1):16–23
Wilson RD, Islam MS (2012) Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacol Rep 64(1):129–139
Zhang S, Yuan L, Zhang L, Li C, Li J (2018) Prophylactic use of troxerutin can delay the development of diabetic cognitive dysfunction and improve the expression of Nrf2 in the Hippocampus on STZ diabetic rats. Behav Neurol 8678539
Acknowledgments
I thank Prof. C. Keck (Philips University, Marburg, Germany) for giving APE the opportunity to work in her laboratory for the production of SeNPs.
Author contribution statement
APE, JON, and CECCE conceived and designed research. APE, SOO, and SOO conducted experiments and analyzed data. APE, SOO, JON, and CECCE wrote the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Funding
This research was supported by the TWAS-DFG 2017 cooperation visit grant and AE-FUNAI Institutional Based research grant (FUNAI/FS/B1/005), both awarded to Azubuike P. Ebokaiwe.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 358 kb)
Rights and permissions
About this article
Cite this article
Ebokaiwe, A.P., Okori, S., Nwankwo, J.O. et al. Selenium nanoparticles and metformin ameliorate streptozotocin-instigated brain oxidative-inflammatory stress and neurobehavioral alterations in rats. Naunyn-Schmiedeberg's Arch Pharmacol 394, 591–602 (2021). https://doi.org/10.1007/s00210-020-02000-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-020-02000-2