Abstract
Rational
Activation of nicotinic acetylcholine receptors has a major neuromodulatory impact on central nervous system function. Beyond acute activation, chronic nicotine intake has long-lasting effects on cortical excitability in animal experiments, caused by receptor up- or down-regulation. Knowledge about the impact of nicotine on cortical excitability in humans, however, is limited.
Objectives
We therefore aimed to explore the effect of nicotine intake on cortical excitability in healthy human smokers and non-smokers.
Methods
The primary motor cortex served as model, and cortical excitability was monitored via transcranial magnetic stimulation (TMS). Corticospinal excitability and intracortical excitability were recorded before and after application of nicotine patch in both groups. Corticospinal excitability was explored by motor threshold and input/output curve (I/O curve), and intracortical excitability was explored by means of paired-pulse TMS techniques (intracortical facilitation (ICF), short-latency intracortical inhibition (SICI), I-wave facilitation and short-latency afferent inhibition (SAI)).
Results
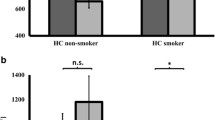
The results show that smokers during nicotine withdrawal display increased corticospinal excitability with regard to the I/O curve (TMS intensity 150 % of resting motor threshold) compared to non-smokers and furthermore enhanced SAI and diminished ICF at the intracortical circuit level. After administration of nicotine, intracortical facilitation in smokers increased, while in non-smokers, inhibition (SICI, SAI) was enhanced.
Conclusion
Our results show that chronic nicotine consumption in smokers alters cortical excitability independent from acute nicotine consumption and that acute nicotine has different effects on motor cortical excitability in both groups.





Similar content being viewed by others
Abbreviations
- ADM:
-
Abductor digiti minimi muscle
- AMT:
-
Active motor threshold
- ICF:
-
Intracortical facilitation
- I/O curves:
-
Input/output curves
- ISI:
-
Interstimulus interval
- MEP:
-
Motor evoked potential
- nAChR:
-
Nicotinic acetylcholine receptors
- RMT:
-
Resting motor threshold
- S1mV:
-
TMS intensity needed to elicit an MEP of 1 mV
- SAI:
-
Short-interval afferent inhibition
- SICI:
-
Short-latency intracortical inhibition
- TMS:
-
Transcranial magnetic stimulation
References
Alkandon M, Braga MF, Pereira EF, Maelicke A, Albuquerque EX (2000) Alpha7 nicotinic acetylcholine receptors and modulation of gabaergic synaptic transmission in the hippocampus. Eur J Pharmacol 393:59–67
Barone JA (1999) Domperidone: a peripherally acting dopamine2-receptor antagonist. Ann Pharmacother 33(4):429–440, Review
Burnashev N (1998) Calcium permeability of ligand-gated channels. Cell Calcium 24(5–6):325–332, Review
Chen R (2000) Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl (9):S26–32
Chevallier S, Nagy F, Cabelguen JM (2006) Cholinergic control of excitability of spinal motoneurones in the salamander. J Physiol 570(Pt 3):525–540
Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD (2010) Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. NeuroImage 52(2):590–599
Cordero-Erausquin M, Marubio LM, Klink R, Changeux JP (2000) Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol Sci 21(6):211–217
Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC (1998) Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol 109(5):397–401
Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC (2000) Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res 135:455–461
Di Lazzaro V, Pilato F, Dileone M, Tonali MA, Ziemann U (2005) Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J Physiol 569:315–323
Di Lazzaro V, Pilato F, Dileone M, Saturno E, Oliviero A, Marra C, Daniele A, Ranieri F, Gainotti G, Tonali PA (2006) In vivo cholinergic circuit evaluation in frontotemporal and Alzheimer dementias. Neurology 66(7):1111–1113
Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, Bria P, Tonali PA, Ziemann U (2007) Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol 118(10):2207–2214
Even N, Cardona A, Soudant M, Corringer PJ, Changeux JP, Cloëz-Tayarani I (2008) Regional differential effects of chronic nicotine on brain alpha 4-containing and alpha 6-containing receptors. Neuroreport 19:1545–1550
Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA (1997) Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci 17(15):5747–5759
Fiore MC, Smith SS, Jorenby DE, Baker TB (1994) The effectiveness of the nicotine patch for smoking cessation. A meta-analysis. JAMA 271(24):1940–1947
Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, Marcolin MA, Rigonatti SP, Silva MT, Paulus W, Pascual-Leone A (2005) Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res 166(1):23–30
Goff DC, Coyle JT (2001) The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry 158(9):1367–1377, Review
Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA (1996) Hippocampal synaptic transmission enhanced by low concentration of nicotine. Nature 383:713–716
Hahn B, Shoaib M, Stolerman IP (2002) Nicotine-induced enhancement of attention in the five-choice serial reaction time task, the influence of task demand. Psychopharmacology 162:129–137
Hogg RC, Raggenbass M, Bertrand D (2003) Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol 147:1–46, Epub 2003 Mar 20. Review
Hosur V, Leppanen S, Abutaha A, Loring RH (2009) Gene regulation of alpha4beta2 nicotinic receptors: microarray analysis of nicotine-induced receptor up-regulation and anti-inflammatory effects. J Neurochem 111(3):848–5
Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U (2002) Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol 545(Pt 1):153–167
Jakobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR (2005) Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Psychiatry 57:56–66
Kawa K (2007) Inhibitory synaptic transmission in area postrema neurons of the rat showing robust presynaptic facilitation mediated by nicotinic ACh receptors. Brain Res 1130(1):83–94
Kleykamp BA, Jennings JM, Eissenberg T (2011) Effects of transdermal nicotine and concurrent smoking on cognitive performance in tobacco-abstinent smokers. Exp Clin Psychopharmacol 19(1):75–84
Korchounov A, Ilic TV, Schwinge T, Ziemann U (2005) Modification of motor cortical excitability by an acetylcholinesterase inhibitor. Exp Brain Res 164:399–405
Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Kumari V, Gray JA, ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, Vythelingum GN, Williams SC, Simmons A, Sharma T (2003) Cognitive effects of nicotine in humans: an fMRI study. NeuroImage 19:1002–1012
Lang N, Hasan A, Sueske E, Paulus W, Nitsche MA (2008) Cortical hypoexcitability in chronic smokers? A transkranial magnetic stimulation study. Neuropsychopharmacology 33:2517–2523
Lang N, Rothkegel H, Reiber H, Hasan A, Sueske E, Tergau F, Ehrenreich H, Wuttke W, Paulus W (2011) Circadian modulation of GABA-mediated cortical inhibition. Cereb Cortex 21(10):2299–2306
Le Novère N, Corringer PJ, Changeux JP (2002) The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J Neurobiol 53(4):447–456, Review
Levin ED, McClernon FJ, Rezvani AH (2006) Nicotinic effects on cognitive function: behavioural characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 184:523–539, Review
Mansvelder HD, van Aerde KI, Couey JJ, Brussaard AB (2006) Nicotinic modulation of neuronal networks: from receptors to cognition. Psychopharmacology (Berl) 184(3–4):292–305, Review
Mansvelder HD, Mertz M, Role LW (2009) Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Semin Cell Dev Biol 20(4):432–440
Miura M, Ishii K, Asoki T, Sumikawa K (2006) Chronic nicotine treatment increases GABA-ergic input to striatal neurons. Neuroreport 17:537–540
Orth M, Münchau A, Rothwell JC (2008) Corticospinal system excitability at rest is associated with tic severity in Tourette syndrome. Biol Psychiatry 64(3):248–251
Parish CL, Nunan J, Finkelstein DI, McNamara FN, Wong JY, Waddington JL, Brown RM, Lawrence AJ, Horne MK, Drago J (2005) Mice lacking the alpha4 nicotinic receptor subunit fail to modulate dopaminergic neuronal arbors and possess impaired dopamine transporter function. Mol Pharmacol 68(5):1376–1386
Potter AS, Newhouse PA (2008) Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol Biochem Behav 88:407–417
Ridding MC, Rothwell JC (1997) Stimulus/response curves as a method of measuring motor cortex excitability in man. Electroencephalogr Clin Neurophysiol 105:340–344
Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W (1999) Magnetic stimulation: motor evoked potentials. Int Fed Clin Neuropysiology Electroencephalogr Clin Neurophysiol Suppl 52:97–103
Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP (2005) Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry 62:649–659
Schwartz RD, Kellar KJ (1983) Nicotinic cholinergic receptor binding sites in the brain: regulation in vivo. Science 220(4593):214–216
Schwenkreis P, Witscher K, Janssen F, Addo A, Dertwinkel R, Zenz M, Malin JP, Tegenthoff M (1999) Influence of the N-methyl-d-aspartate antagonist memantine on human motor cortex excitability. Neurosci Lett 270(3):137–140
Spirt MJ, Chan W, Thieberg M, Sachar DB (1992) Neuroleptic malignant syndrome induced by domperidone. Dig Dis Sci 37(6):946–948
Strenge H, Schmidt G, Niederberger U, Porschke H, Schütz HW (1996) Effects of nicotine gum on F waves in non-smokers. Funct Neurol 11(4):179–185
Thiel CM, Zilles K, Fink GR (2005) Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology 30:810–820
Turner TJ (2004) Nicotine enhancement of dopamine release by a calcium-dependent increase in the size of the readily releasable pool of synaptic vesicles. J Neurosci 24(50):11328–11336
Wall A, Gong ZH, Johnson AE, Meyerson B, Zhang X (2000) Cross-tolerance in drug, response and differential changes in central nicotinic and N-methyl-d-aspartate receptor binding following chronic treatment with either (+)- or (−)-nicotine. Psychopharmacology 148:186–195
Wang BW, Liao WN, Chang CT, Wang SJ (2006) Facilitation of glutamate release by nicotine involves the activation of a Ca2+/calmodulin signaling pathway in rat prefrontal cortex nerve terminals. Synapse 59(8):491–501
Wang F, Chen H, Steketee JD, Sharp BM (2007) Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacology 32(1):103–109
Ziemann U (2004) TMS induced plasticity in human cortex. Rev Neurosci 15(4):253–266
Ziemann U, Lönnecker S, Paulus W (1995) Inhibition of human motor cortex by ethanol. A transcranial magnetic stimulation study. Brain 118:1437–1446
Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W (1998a) Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol 511:181–190
Ziemann U, Chen R, Cohen LG, Hallett M (1998b) Dextromethorphan decreases the excitability of the human motor cortex. Neurology 51(5):1320–1324
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG grant NI683/4-1 ‘Towards risk prediction of nicotine dependency by exploring individual limits of cortical neuroplasticity in humans’ and ‘Impact of the nicotinergic alpha7 receptor on cortical plasticity in smokers and non-smokers’) within the DFG priority program ‘Nicotine: Molecular and Physiological Effects in Central Nervous System’.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S 1
(DOC 40 kb)
Table S 2
(DOC 82 kb)
Figure Online Resource 2
Displays the I/O curve for 10 healthy non-smoking subjects at baseline, 2 hours after administration of 20 mg domperidone and again 4 hours after administration of a second dosage domperidone (6 hours post, relating to baseline). Shown are the graphs with the standardized MEP-amplitudes (y-axis) plotted against the respective stimulation intensity (x-axis). Hereby RMT signifies resting motor threshhold. There is no significant difference between the MEP-amplitudes at baseline and the MEP-amplitudes 2 and 6 hours after baseline measurements and domperidone ingestion (Students t-test, paired, two-tailed, p < 0.05). Error bars indicate standard error of mean. (PPT 137 kb)
Figure Online Resource 3
Displays MEP-amplitudes at different timepoints. TMS intensity was determined to elicit an MEP of about 1 mV at baseline and kept constant throughout the experiment. Shown are the graphs with motor-evoked potential (MEP) on the y-axis at different timepoints after administration of domperidone (x-axis). There is no significant difference between the MEP-amplitude before and after administration of domperidone (2 hours and 6 hours) (Students t-test, paired, two-tailed, p < 0.05). Error bars indicate standard error of mean. (PPT 108 kb)
Figure Online Resource 4
Displays short latency afferent inhibition (SAI) at an interstimulus interval of 20 ms for 6 healthy non-smokers at baseline, 2 hours after administration of 20 mg domperidone and again 4 hours after administration of a second dosage domperidone (6 hours post, relating to baseline). Shown are the graphs with the standardized MEP-amplitude (y-axis) plotted against the timepoints (x-axis; baseline, 2 hours post, 6 hours post). There is no significant difference between the MEP-amplitude at baseline and the MEP-amplitude 2 and 6 hours after baseline measurements and domperidone ingestion (Students t-test, paired, two-tailed, p < 0.05). Error bars indicate standard error of mean. (PPT 114 kb)
Figure Online Resource 5
Displays short interval intracortical inhibition (SICI) and intracortical facilitation (ICF) of 10 healthy non-smoking subjects at baseline, 2 hours after administration of 20 mg domperidone and again 4 hours after administration of a second dosage domperidone (6 hours post, relating to baseline). Shown are the graphs with the standardized MEP-amplitudes (y-axis) plotted against the respective interstimulus intervals ( x-axis; 2 ms, 3 ms, 7 ms, 10 ms, 15 ms). There is no significant difference between the MEP-amplitudes at baseline and the MEP-amplitudes 2 and 6 hours after baseline measurements and domperidone ingestion (Students t-test, paired, two-tailed, p < 0.05). Error bars indicate standard error of mean. (PPT 137 kb)
Figure Online Resource 6
Displays the i-wave facilitation of 6 healthy non-smoking subjects at different interstimulus intervals (ISIs) at baseline, 2 hours after administration of 20 mg domperidone and again 4 hours after administration of a second dosage domperidone (6 hours post, relating to baseline). Shown are the graphs with the standardized MEP-amplitudes (y-axis) plotted against the respective interstimulus intervals (x-axis). There is no significant difference between the MEP-amplitudes at baseline and the MEP-amplitudes 2 and 6 hours after baseline measurements and domperidone ingestion (Students t-test, paired, two-tailed, p < 0.05). Error bars indicate standard error of mean. (PPT 150 kb)
Rights and permissions
About this article
Cite this article
Grundey, J., Freznosa, S., Klinker, F. et al. Cortical excitability in smoking and not smoking individuals with and without nicotine. Psychopharmacology 229, 653–664 (2013). https://doi.org/10.1007/s00213-013-3125-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3125-6