Abstract
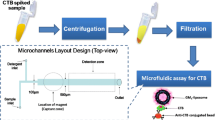
Fluorescence and electrochemical microfluidic biosensors were developed for the detection of cholera toxin subunit B (CTB) as a model analyte. The microfluidic devices were made from polydimethylsiloxane (PDMS) using soft lithography from silicon templates. The polymer channels were sealed with a glass plate and packaged in a polymethylmethacrylate housing that provided leakproof sealing and a connection to a syringe pump. In the electrochemical format, an interdigitated ultramicroelectrode array (IDUA) was patterned onto the glass slide using photolithography, gold evaporation and lift-off processes. For CTB recognition, CTB-specific antibodies were immobilized onto superparamagnetic beads and ganglioside GM1 was incorporated into liposomes. The fluorescence dye sulforhodamine B (SRB) and the electroactive compounds potassium hexacyanoferrate (II)/hexacyanoferrate (III) were used as detection markers that were encapsulated inside the liposomes for the fluorescence and electrochemical detection formats, respectively. Initial optimization experiments were carried out by applying the superparamagnetic beads in microtiter plate assays and SRB liposomes before they were transferred to the microfluidic systems. The limits of detection (LoD) of both assay formats for CTB were found to be 6.6 and 1.0 ng mL−1 for the fluorescence and electrochemical formats, respectively. Changing the detection system was very easy, requiring only the synthesis of different marker-encapsulating liposomes, as well as the exchange of the detection unit. It was found that, in addition to a lower LoD, the electrochemical format assay showed advantages over the fluorescence format in terms of flexibility and reliability of signal recording.






Similar content being viewed by others
Abbreviations
- AP:
-
alkaline phosphatase
- BSA:
-
bovine serum albumin
- DPPC:
-
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- DPPE:
-
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine
- DPPG:
-
1,2-dipalmitoyl-sn-glycero-3-[phospho-rac-(1-glycerol)], sodium salt
- HEPES:
-
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- HSS:
-
HEPES-saline-sucrose
- IMS:
-
immunomagnetic separation
- OG:
-
n-octyl-β-D-glucopyranoside
- PBS:
-
phosphate-buffered saline
- SRB:
-
sulforhodamine B
- TBS:
-
Tris-buffered saline
References
Tsai YC, Jen HP, Lin KW, Hsieh YZ (2006) Fabrication of microfluidic devices using dry film photoresist for microchip capillary electrophoresis. J Chromatogr A 1111:267–271
Das C, Fredrickson CK, Xia Z, Fan ZH (2007) Device fabrication and integration with photodefinable microvalves for protein separation. Sens Actuators A 134:271–277
Gao Y, Lin FYH, Hu G, Sherman PM, Li D (2005) Development of a novel electrokinetically driven microfluidic immunoassay for the detection of Helicobacter pylori. Anal Chim Acta 543:109–116
Zhang Q, Zhu L, Feng H, Ang S, Chau FS, Liu WT (2006) Microbial detection in microfluidic devices through dual staining of quantum dots-labeled immunoassay and RNA hybridization. Anal Chim Acta 556:171–177
Nashida N, Satoh W, Fukuda J, Suzuki H (2007) Electrochemical immunoassay on a microfluidic device with sequential injection and flushing functions. Biosens Bioelectron 22:3167–3173
Tung YC, Zhang M, Lin CT, Kurabayashi K, Skerlos SJ (2004) PDMS-based opto-fluidic micro flow cytometer with two-color, multi-angle fluorescence detection capability using PIN photodiodes. Sens Actuators B 98:356–367
Oleschuk RD, Harrison DJ (2000) Analytical microdevices for mass spectrometry. Trends Anal Chem 19:379–388
Vollmer S, Wells JA, Bergman T, Jornvall H (2007) Microfluidic electrocapture interfaced with electrospray mass spectrometry. Int J Mass Spectrom 259:73–78
Lagally ET, Simpson PC, Mathies RA (2000) Monolithic integrated microfluidic DNA amplification and capillary electrophoresis analysis system. Sens Actuators B 63:138–146
Taylor P, Manage DP, Helmle KE, Zhang Y, Glerum DM, Backhouse CJ (2005) Analysis of mitochondrial DNA in microfluidic systems. J Chromatogr B 822:78–84
Hui WC, Yobas L, Samper VD, Heng CK, Liw S, Ji H, Chen Y, Cong L, Li J, Lim TM (2007) Microfluidic systems for extracting nucleic acids for DNA and RNA analysis. Sens Actuators A 133:335–339
Sadani Z, Wacogne B, Pieralli C, Roux C, Gharbi T (2005) Microsystems and microfluidic device for single oocyte transportation and trapping: toward the automation of in vitro fertilizing. Sens Actuators A 121:364–372
Chen X, Cui D, Liu C, Li H, Chen J (2007) Continuous flow microfluidic device for cell separation, cell lysis and DNA purification. Anal Chim Acta 584:237–243
Ahn-Yoon S, DeCory TR, Baeumner AJ, Durst RA (2003) Ganglioside-liposome immunoassay for the ultrasensitive detection of cholera toxin. Anal Chem 75:2256–2261
Chaicumpa W, Srimanote P, Sakolvaree Y, Kalampaheti T, Chongsanguan M, Tapchaisri P, Eampokalap B, Moolasart P, Nair GB, Echeverria P (1998) Rapid diagnosis of cholera caused by Vibrio cholerae O139. J Clin Microbiol 36:3595–3600
Ngundi MM, Taitt CR, McMurry SA, Kahne D, Ligler FS (2006) Detection of bacterial toxins with monosaccharide arrays. Biosens Bioelectron 21:1195–1201
Charles PT, Velez F, Soto CM, Goldman ER, Martin BD, Ray RI, Taitt CR (2006) A galactose polyacrylate-based hydrogel scaffold for the detection of cholera toxin and staphylococcal enterotoxin B in a sandwich immunoassay format. Anal Chim Acta 578:2–10
Oxoid Diagnostic Reagents (2004) Manufacturer’s instructions for VET-RPLA toxin detection kit. Oxoid Diagnostic Reagents, Basingstoke, UK
Svennerholm AM, Wiklund G (1983) Rapid GM1-enzyme-linked immunosorbent assay with visual reading for identification of Escherichia coli heat-labile enterotoxin. J Clin Microbiol 17:596–600
Edwards KA, March JC (2007) GM1-functionalized liposomes in a microtiter plate assay for cholera toxin in Vibrio cholerae culture samples. Anal Biochem 368:39–48
Ramamurthy T, Bhattacharya SK, Uesaka Y, Horigome K, Paul M, Sen D, Pal SC, Takeda T, Takeda Y, Nair GB (1992) Evaluation of the bead enzyme-linked immunosorbent assay for detection of cholera toxin directly from stool specimens. J Clin Microbiol 30:1783–1786
McDowall J (2005) Cholera toxin (webpage). http://www.ebi.ac.uk/interpro/potm/2005_9/Page1.htm, accessed 7 Jan 2008
Zaytseva NV, Goral VN, Montagna RA, Baeumner AJ (2005) Development of a microfluidic biosensor module for pathogen detection. Lab Chip 5:805–811
Zaytseva NV, Montagna RA, Baeumner AJ (2005) Microfluidic biosensor for the serotype-specific detection of Dengue virus RNA. Anal Chem 77:7520–7527
Goral VN, Zaytseva NV, Baeumner AJ (2006) Electrochemical microfluidic biosensor for the detection of nucleic acid sequences. Lab Chip 6:414–421
León LD, Siverio F, Rodríguez A (2006) Detection of Clavibacter michiganensis subsp. michiganensis in tomato seeds using immunomagnetic separation. J Microbiol Methods 67:141–149
Soulier SH, Guillot E (1999) An immunomagnetic separation polymerase chain reaction assay for rapid and ultra-sensitive detection of Cryptosporidium parvum in drinking water. FEMS Microbiol Lett 176:285–289
Fu Z, Rogelj S, Kieft TL (2005) Rapid detection of Escherichia coli O157:H7 by immunomagnetic separation and real-time PCR. Int J Food Microbiol 99:47–57
Liu Y, Che Y, Li Y (2001) Rapid detection of Salmonella typhimurium using immunomagnetic separation and immuno-optical sensing method. Sens Actuators B 72:214–218
Gehring AG, Irwin PL, Reed SA, Tu SI, Andreotti PE, Tafti HA, Handley RS (2004) Enzyme-linked immunomagnetic chemiluminescent detection of Escherichia coli O157:H7. J Immunol Methods 293:97–106
Seo KH, Brackett RE, Frank JF (1998) Rapid detection of Escherichia coli O157:H7 using immunomagnetic flow cytometry in ground beef, apple juice, and milk. Int J Food Microbiol 44:115–123
Hibi K, Abe A, Ohashi E, Mitsubayashi K, Ushio H, Hayashi T, Ren H, Endo H (2006) Combination of immunomagnetic separation with flow cytometry for detection of Listeria monocytogenes. Anal Chim Acta 573–574:158–163
Kwakye S, Baeumner A (2003) A microfluidic biosensor based on nucleic acid sequence recognition. Anal Bioanal Chem 376:1062–1068
Bartlett GR (1959) Phosphorus assay in column chromatography. J Biol Chem 234:466–468
Nichols KP, Ferullo JR, Baeumner AJ (2006) Recirculating microfluidic mixer. Lab Chip 6:242–246
Kwakye S, Goral VN, Baeumner AJ (2006) Electrochemical microfluidic biosensor for nucleic acid detection with integrated minipotentiostat. Biosens Bioelectron 21:2217–2223
Wang J (2002) Electrochemical detection for microscale analytical systems: a review. Talanta 56:223–231
Acknowledgements
The authors thank Dr. John March of Cornell University for their use of the inverted fluorescence microscope. We thank Barbara Leonard for help with the preparation of the liposomes. We thank Dr. Natalya Zaytseva for helpful advice on microfluidic analysis. We also thank John Connelly and Dr. Sam Nugen for microfabrication training. This research was supported in part by the Cornell University Agricultural Experiment Station federal formula funds, Project No. 123-464, received from Cooperative State Research, Education and Extension Service, US Department of Agriculture. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture. This work was performed in part at the Cornell Nanofabrication Facility, a member of the National Nanofabrication Users Network, which is supported by the National Science Foundation (grant ECS-0335765). This research was also supported in part by the Thailand Research Fund (TRF) through the RGJ-PhD program in Thailand, and the Commission on Higher Education, Ministry of Education, Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bunyakul, N., Edwards, K.A., Promptmas, C. et al. Cholera toxin subunit B detection in microfluidic devices. Anal Bioanal Chem 393, 177–186 (2009). https://doi.org/10.1007/s00216-008-2364-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-008-2364-6