Abstract
The development of a simple sensor (9NL27-Zn) based on DNAzyme and PCR and aimed at the detection of low concentrations of zinc (II) ions is described. A specific Zn(II)-dependent DNAzyme (9NL27) with DNA-cleaving activity was employed. In the presence of zinc (II), the DNAzyme hydrolyzed DNA substrate into two pieces (5′ and 3′ fragments), forming 3′-terminal hydroxyl in the 5′ fragment and 5′-phosphate in the 3′ fragments. Subsequently, the 5′ fragment left the DNAzyme and bound a short DNA template. The 5′ fragment was used as a primer and extended a single-stranded full-length template by Taq polymerase. Finally, this full-length template was amplified by PCR. The amplified products had a quantitative relationship with Zn(II) concentration. Under our experimental conditions, the DNA sensor showed sensitivity (10 nM) and high specificity for zinc ion detection. After improvement of the DNA sensor, the detection limit can reach 1 nM. The simple DNA sensor may become a DNA model for the detection of trace amounts of other targets.

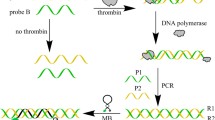
The general principle of a 9NL27-Zn sensor by the combination of a Zn2+-dependent DNAzyme and PCR. The red arrowhead indicates the cleavage site of DNA substrate




Similar content being viewed by others
References
Breaker RR, Joyce GF (1994) A DNA enzyme that cleaves RNA. Chem Biol 1:223–229
Santoro W, Joyce GF (1997) A general purpose RNA-cleaving DNA enzyme. Proc Natl Acad Sci U S A 94:4262–4266
Roth A, Breaker RR (1998) An amino acid as a cofactor for a catalytic polynucleotide. Proc Natl Acad Sci U S A 95:6027–6031
Cruz RPG, Withers JB, Li Y (2004) Dinucleotide junction cleavage versatility of 8-17 deoxyribozyme. Chem Biol 11:57–67
Liu J, Brown AK, Meng X, Cropek DM, Istok JD, Watson DB, Lu Y (2007) A catalytic beacon sensor for uranium with parts-pertrillion sensitivity and millionfold selectivity. Proc Natl Acad Sci U S A 104:2056–2061
Willner I, Shlyahovsky B, Zayats M, Willner B (2008) DNAzymes for sensing, nanobiotechnology and logic gate applications. Chem Soc Rev 37:1153–1165
Liu J, Cao Z, Lu Y (2009) Functional nucleic acid sensors. Chem Rev 109:1948–1998
Dai N, Kool ET (2011) Fluorescent DNA-based enzyme sensors. Chem Soc Rev 40:5756–5770
Zhang X, Kong R, Lu Y (2011) Metal ion sensors based on DNAzymes and related DNA molecules. Annu Rev Anal Chem 4:105–128
Juskowiak B (2011) Nucleic acid-based fluorescent probes and their analytical potential. Anal Bioanal Chem 399:3157–3176
Miao X, Ling L, Cheng D, Shuai X (2012) A highly sensitive sensor for Cu2+ with unmodified gold nanoparticles and DNAzyme by using the dynamic light scattering technique. Analyst 137:3064–3069
Zhang H, Jiang B, Xiang Y, Su J, Chai Y, Yuan R (2011) DNAzyme-based highly sensitive electronic detection of lead via quantum dot-assembled amplification labels. Biosens Bioelectron 28:135–138
Prasad AS (2013) Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr 4:176–190
Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME (2012) Zinc and human health: an update. Arch Toxicol 86:521–534
Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ (2012) Zinc and liver disease. Nutr Clin Pract 27:8–20
Mocchegiani E, Malavolta M, Costarelli L, Giacconi R, Cipriano C, Piacenza F, Tesei S, Basso A, Pierpaoli S, Lattanzio F (2010) Zinc, metallothioneins and immunosenescence. Proc Nutr 69:290–299
Chyan W, Zhang DY, Lippard SJ, Radford RJ (2014) Reaction-based fluorescent sensor for investigating mobile Zn2+ in mitochondria of healthy versus cancerous prostate cells. Proc Natl Acad Sci USA Advance Article
Liu Y, Fei Q, Shan H, Cui M, Liu Q, Feng G,Huan Y (2014) A novel fluorescent ‘off-on-off’ probe for relay recognition of Zn2+ and Cu2+ derived from N,N-bis(2-pyridylmethyl)amine. Analyst Advance Article
Chen M, Lv X, Liu Y, Zhao Y, Liu J, Wang P, Guo W (2011) An 2-(2’-aminophenyl)benzoxazole-based OFF-ON fluorescent chemosensor for Zn2+ in aqueous solution. Org Biomol Chem 9:2345–2349
Ashokkumar P, Ramakrishnan VT, Ramamurthy P (2011) Photoinduced electron transfer (PET) based Zn2+ fluorescent probe: transformation of turn-on sensors into ratiometric ones with dual emission in acetonitrile. J Phys Chem A 115:14292–14299
Buccella D, Horowitz JA, Lippard SJ (2011) Understanding zinc quantification with existing and advanced ditopic fluorescent Zinpyr sensors. J Am Chem Soc 133:4101–4114
Chandra M, Sachdeva A, Silverman SK (2009) DNA-catalyzed sequence-specific hydrolysis of DNA. Nat Chem Biol 5:718–720
Xiao Y, Allen EC, Silverman SK (2011) Merely two mutations switch a DNA-hydrolyzing deoxyribozyme from heterobimetallic (Zn2+/Mn2+) to monometallic (Zn2+-only) behavior. Chem Commun 47:1749–1751
Gu H, Furukawa K, Weinberg Z, Berenson DF, Breaker RR (2013) Small, highly active DNAs that hydrolyze DNA. J Am Chem Soc 135:9121–9129
Hindson CM, Chevillet JR, Briggs HA, Gallichotte EN, Ruf IK, Hindson BJ, Vessella RL, Tewari M (2013) Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods 10:1003–1005
Wang F, Wu Z, Lu Y, Wang J, Jiang J, Yu R (2010) A label-free DNAzyme sensor for lead(II) detection by quantitative polymerase chain reaction. Anal Biochem 405:168–173
Todd AV, Fuery CJ, Impey HL, Applegate TL, Haughton MA (2000) DzyNA-PCR use of DNAzymes to detect and quantify nucleic acid sequences in a real-time fluorescent format. Clin Chem 46:625–630
Acknowledgments
This research was supported by the Fundamental Research Funds for Jilin University (200903096) and National Natural Science Foundation of China (31100573).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1911 kb)
Rights and permissions
About this article
Cite this article
Xu, J., Sun, Y., Sheng, Y. et al. Engineering a DNA-cleaving DNAzyme and PCR into a simple sensor for zinc ion detection. Anal Bioanal Chem 406, 3025–3029 (2014). https://doi.org/10.1007/s00216-014-7732-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7732-9