Abstract
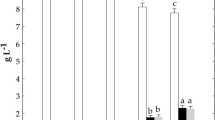
Fermentable, oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) are a class of carbohydrates poorly digested that may trigger the symptoms of Irritable Bowel Syndrome (IBS) and Non-Celiac Gluten Sensitivity (NCGS). The effects of sourdough fermentation on FODMAPs and organic acids were studied during the sourdough propagation and bread making. The concentrations of organic acids were higher for the first steps of propagation and became stable for final steps. All FODMAPs were significantly reduced during the propagation, except polyols. Sucrose, fructose and glucose were wholly degraded for the first step of fermentation. The other carbohydrates had their concentrations reduced after the fourth backslopping step. Sourdough bread presented the higher level of organic acids and polyols, and lower content of fructans, sucrose, fructose and glucose than bread fermented by Saccharomyces cerevisiae. The fructan reduction was from 69 to 75%, indicating that sourdough fermentation can be applied for producing low-FODMAP wheat bakery products.






Similar content being viewed by others
References
Gobbetti M, Rizzello CG, Di Cagno R, De Angelis M (2014) How the sourdough may affect the functional features of leavened baked goods. Food Microbiol 37:30–40
Minervini F, De Angelis M, Di Cagno R, Gobbetti M (2014) Ecological parameters influencing microbial diversity and stability of traditional sourdough. Int J Food Microbiol 171:136–146
Pétel C, Onno B, Prost C (2017) Sourdough volatile compounds and their contribution to bread: a review. Trends Food Sci Technol 59:105–123
Rinaldi M, Paciulli M, Caligiani A, Scazzina F, Chiavaro E (2017) Sourdough fermentation and chestnut flour in gluten-free bread: a shelf-life evaluation. Food Chem 224:144–152
Torrieri E, Pepe O, Ventorino V, Masi P, Cavella S (2014) Effect of sourdough at different concentrations on quality and shelf life of bread. LWT Food Sci Technol 56(2):508–516
Gänzle MG (2014) Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol 37:2–10
Van Kerrebroeck S, Maes D, De Vuyst L (2017) Sourdoughs as a function of their species diversity and process conditions, a meta-analysis. Trends Food Sci Technol 68:152–159
Dertli E, Mercan E, Arici M, Yilmaz MT, Sağdiç O (2016) Characterisation of lactic acid bacteria from Turkish sourdough and determination of their exopolysaccharide (EPS) production characteristics. LWT Food Sci Technol 71:116–124
Muir JG et al (2009) Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography (HPLC). J Agric Food Chem 57(2):554–565
Menezes LAA et al (2018) Effects of sourdough on FODMAPs in bread and potential outcomes on irritable bowel syndrome patients and healthy subjects. Front Microbiol 9(AUG):1–7
Brandt LJ (2009) An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol 104(Suppl 1):S1–S35
Lovell RM, Ford AC (2012) Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 10(7):712–721
De Giorgio R, Volta U, Gibson PR (2016) Sensitivity to wheat, gluten and FODMAPs in IBS: facts or fiction? Gut 65(1):169–178
Tuck CJ, Muir JG, Barrett JS, Gibson PR (2014) Fermentable oligosaccharides, disaccharides, monosaccharides and polyols: role in irritable bowel syndrome. Expert Rev Gastroenterol Hepatol 8(7):819–834
Zannini E, Arendt EK (2018) Low FODMAPs and gluten-free foods for irritable bowel syndrome treatment: lights and shadows. Food Res Int 110:33–41
Dieterich W et al (2018) Influence of low FODMAP and gluten-free diets on disease activity and intestinal microbiota in patients with non-celiac gluten sensitivity. Clin Nutr. https://doi.org/10.1016/j.clnu.2018.03.017
Skodje GI, Sarna VK, Minelle IH, Rolfsen KL, Muir JG, Gibson PR, Veierød MB, Henriksen C, Lundin KE (2018) Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology 154:529–539
Henggeler JC, Veríssimo M, Ramos F (2017) Non-coeliac gluten sensitivity: a review of the literature. Trends Food Sci Technol 66:84–92
Biesiekierski JR et al (2013) No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 145(2):320–328.e3
Halmos EP et al (2015) Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 64(1):93–100
Biesiekierski JR et al (2011) Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J Hum Nutr Diet 24(2):154–176
Shewry PR, Hey SJ (2015) The contribution of wheat to human diet and health. Food Energy Secur 4(3):178–202
Ziegler JU et al (2016) Wheat and the irritable bowel syndrome—FODMAP levels of modern and ancient species and their retention during bread making. J Funct Foods 25:257–266
Lhomme E et al (2015) Lactic acid bacterium and yeast microbiotas of sixteen French traditional sourdoughs. Int J Food Microbiol 215:161–170
Li Z, Li H, Bian K (2016) Microbiological characterization of traditional dough fermentation starter (Jiaozi) for steamed bread making by culture-dependent and culture-independent methods. Int J Food Microbiol 234:9–14
AACC (2010) Approved methods of analysis (11th ed.). Method 02-31.01 titratable acidity. AACC International, St. Paul
Molognoni L, Daguer H, de Sá Ploêncio LA, De Dea Lindner J (2018) A multi-purpose tool for food inspection: simultaneous determination of various classes of preservatives and biogenic amines in meat and fish products by LC-MS. Talanta 178:1053–1066
Molognoni L, De Sá Ploêncio LA, Valese AC, De Dea Lindner J, Daguer H (2016) A simple and fast method for the inspection of preservatives in cheeses and cream by liquid chromatography-electrospray tandem mass spectrometry. Talanta 147:370–382
Vrancken G, Rimaux T, Weckx S, Leroy F, De Vuyst L (2011) Influence of temperature and backslopping time on the microbiota of a type I propagated laboratory wheat sourdough fermentation. Appl Environ Microbiol 77(8):2716–2726
Ercolini D et al (2013) Microbial ecology dynamics during rye and wheat sourdough preparation. Appl Environ Microbiol 79(24):7827–7836
Scheirlinck I et al (2007) Influence of geographical origin and flour type on diversity of lactic acid bacteria in traditional belgian sourdoughs. Appl Environ Microbiol 73(19):6262–6269
Van Der Meulen R et al (2007) Population dynamics and metabolite target analysis of lactic acid bacteria during laboratory fermentations of wheat and spelt sourdoughs. Appl Environ Microbiol 73(15):4741–4750
Minervini F, Lattanzi A, De Angelis M, Celano G, Gobbetti M (2015) House microbiotas as sources of lactic acid bacteria and yeasts in traditional Italian sourdoughs. Food Microbiol 52:66–76
Vogelmann SA, Seitter M, Singer U, Brandt MJ, Hertel C (2009) Adaptability of lactic acid bacteria and yeasts to sourdoughs prepared from cereals, pseudocereals and cassava and use of competitive strains as starters. Int J Food Microbiol 130(3):205–212
Liu T et al (2016) Prevalence and diversity of lactic acid bacteria in Chinese traditional sourdough revealed by culture dependent and pyrosequencing approaches. LWT Food Sci Technol 68:91–97
Gobbetti M, Minervini F, Pontonio E, Di Cagno R, De Angelis M (2016) Drivers for the establishment and composition of the sourdough lactic acid bacteria biota. Int J Food Microbiol 239:3–18
De Vuyst L et al (2014) Microbial ecology of sourdough fermentations: diverse or uniform? Food Microbiol 37:11–29
Ventimiglia G et al (2015) Codominance of Lactobacillus plantarum and obligate heterofermentative lactic acid bacteria during sourdough fermentation. Food Microbiol 51:57–68
Harth H, Van Kerrebroeck S, De Vuyst L (2016) Community dynamics and metabolite target analysis of spontaneous, backslopped barley sourdough fermentations under laboratory and bakery conditions. Int J Food Microbiol 228:22–32
Van Kerrebroeck S, Bastos FCC, Harth H, De Vuyst L (2016) A low pH does not determine the community dynamics of spontaneously developed backslopped liquid wheat sourdoughs but does influence their metabolite kinetics. Int J Food Microbiol 239:54–64
Weckx S et al (2010) Lactic acid bacteria community dynamics and metabolite production of rye sourdough fermentations share characteristics of wheat and spelt sourdough fermentations. Food Microbiol 27(8):1000–1008
Bartkiene E et al (2017) The contribution of P. acidilactici, L. plantarum, and L. curvatus starters and l-(+)-lactic acid to the acrylamide content and quality parameters of mixed rye—wheat bread. LWT Food Sci Technol 80:43–50
Dagnas S, Gauvry E, Onno B, Membré J-M (2015) Quantifying effect of lactic, acetic, and propionic acids on growth of molds isolated from spoiled bakery products. J Food Prot 78(9):1689–1698
Nilsson U, Öste R, Jägerstad M (1987) Cereal fructans: hydrolysis by yeast invertase, in vitro and during fermentation. J Cereal Sci 6(1):53–60
Struyf N et al (2017) Establishing the relative importance of damaged starch and fructan as sources of fermentable sugars in wheat flour and whole meal bread dough fermentations. Food Chem 218:89–98
Gobbetti M, De Angelis M, Corsetti A, Di Cagno R (2005) Biochemistry and physiology of sourdough lactic acid bacteria. Trends Food Sci Technol 16(1–3):57–69
Henström M et al (2018) Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut 67(2):263–270
Marques WL, Raghavendran V, Stambuk BU, Gombert AK (2015) Sucrose and Saccharomyces cerevisiae: a relationship most sweet. FEMS Yeast Res 16(1):1–16
Saulnier DMA, Molenaar D, De Vos WM, Gibson GR, Kolida S (2007) Identification of prebiotic fructooligosaccharide metabolism in Lactobacillus plantarum WCFS1 through microarrays. Appl Environ Microbiol 73(6):1753–1765
Kunová G, Rada V, Lisová I, Ročková Š, Vlková E (2011) In vitro fermentability of prebiotic oligosaccharides by Lactobacilli. Czech J Food Sci 29(2):49–54
Gänzle MG, Follador R (2012) Metabolism of oligosaccharides and starch in lactobacilli: a review. Front Microbiol 3(SEP):1–15
Loponen J, Gänzle M (2018) Use of sourdough in low FODMAP baking. Foods 7(7):96
Teixeira JS, McNeill V, Gänzle MG (2012) Levansucrase and sucrose phoshorylase contribute to raffinose, stachyose, and verbascose metabolism by lactobacilli. Food Microbiol 31(2):278–284
Tieking M, Ehrmann MA, Vogel RF, Gänzle MG (2005) Molecular and functional characterization of a levansucrase from the sourdough isolate Lactobacillus sanfranciscensis TMW 1.392. Appl Microbiol Biotechnol 66(6):655–663
Carević M et al (2016) Selection of lactic acid bacteria strain for simultaneous production of α- and β-galactosidases. Zast Mater 57(2):265–273
Tsujikawa Y, Nomoto R, Osawa R (2013) Difference in degradation patterns on inulin-type fructans among strains of Lactobacillus delbrueckii and Lactobacillus paracasei. Biosci Microbiota Food Health 32(4):157–165
Verspreet J, Dornez E, van den Ende W, Delcour JA, Courtin CM (2015) Cereal grain fructans: structure, variability and potential health effects. Trends Food Sci Technol 43(1):32–42
Fraberger V, Call L, Domig KJ, Amico SD (2018) Applicability of yeast fermentation to reduce fructans and other FODMAPs. Nutrients 10(9):1247. https://doi.org/10.3390/nu10091247
De Vuyst L, Vrancken G, Ravyts F, Rimaux T, Weckx S (2009) Biodiversity, ecological determinants, and metabolic exploitation of sourdough microbiota. Food Microbiol 26(7):666–675
Otgonbayar GE, Eom HJ, Kim BS, Ko JH, Han NS (2011) Mannitol production by Leuconostoc citreum KACC 91348P isolated from kimchi. J Microbiol Biotechnol 21(9):968–971
Weckx S et al (2010) Community dynamics of bacteria in sourdough fermentations as revealed by their metatranscriptome. Appl Environ Microbiol 76(16):5402–5408
Arendt EK, Ryan LAM, Dal Bello F (2007) Impact of sourdough on the texture of bread. Food Microbiol 24(2):165–174
Costabile A et al (2014) Effect of breadmaking process on in vitrogut microbiota parameters in irritable bowel syndrome. PLoS One. https://doi.org/10.1371/journal.pone.0111225
Singh RS, Chauhan K, Kennedy JF (2017) A panorama of bacterial inulinases: production, purification, characterization and industrial applications. Int J Biol Macromol 96:312–322
Paludan-Müller C, Gram L, Rattray FP (2002) Purification and characterisation of an extracellular fructan b-fructosidase from a Lactobacillus pentosus strain isolated from fermented fish. Syst Appl Microbiol 25:13–20
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Menezes, L.A.A., Molognoni, L., de Sá Ploêncio, L.A. et al. Use of sourdough fermentation to reducing FODMAPs in breads. Eur Food Res Technol 245, 1183–1195 (2019). https://doi.org/10.1007/s00217-019-03239-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03239-7