Abstract
In the case of biotic and abiotic stresses, natural defence mechanisms are triggered in plants. The mechanisms are based mainly on enzymatic or non-enzymatic intracellular reactions. Changes in the quantitative profile of 12 phenolic acids, total bound phenolic acids, total free phenolic acids and antioxidant activity in grain of 23 winter wheat genotypes exposed to stress (artificial inoculation with spores of fungi from the genus Fusarium or chemical protection) were analysed. Concentrations of ergosterol confirm significantly higher levels of contamination with microscopic fungi in inoculated wheat samples in comparison to the control and chemically protected samples. The highest concentrations of bound phenolic acids were recorded in inoculated samples while the control and chemically protected samples did not differ significantly. Chromatographic analysis of contents of 12 bound phenolic acids showed that significant differences were found for contents of ferulic, synapic, t-cinnamic and 4-hydroxybenzoic acids in experimental variants. Stepwise discriminatory analysis based on the contents of selected bound phenolic acids antioxidant activity and free phenolic acids led to a complete separation of the investigated populations of the experimental groups. The statistical analysis confirmed that free phenolic acids are the most significant factors in resistance mechanisms as the first line of defence together with their antioxidant action, followed by the synergistic effect of exogenous bound phenolic acids: ferulic, synapic, t-cinnamic and 4-hydroxybenzoic acids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crop plants during the vegetation period are exposed to the action of biotic and abiotic stresses. The easiest and cheaper method to prevent losses caused by both biotic and abiotic stresses is to grow cultivars resistant to diseases and tolerating diverse environmental factors [1]. Fusarium head blight is observed in all cereals grown in central and eastern Europe; however, it is the greatest threat for wheat and maize cultivation [2, 3]. In Poland, fusarium head blight in wheat is caused mainly by F. culmorum, F. graminearum and F. avenaceum [4]. Fungal diseases cause direct reduction of yields, while deterioration in grain quality results from the formation of various types of fungal metabolites [5]. The most important and most frequent mycotoxins found in Poland in cereal grain include type B trichothecenes, primarily deoxynivalenol and nivalenol [4].
In conventional farming systems, wheat is exposed to fungal infections inducing stress caused both by the presence of the pathogen and mycotoxins it secretes to plant tissues. However, to avoid or reduce infection, chemical pesticides are applied, also leading to stress in plants, even healthy ones. As a result in the case of fungal infection and the application of fungicides, we deal with a double stress: abiotic (fungicide, mycotoxins) and biotic (the presence of a pathogen).
In such a situation, natural defence mechanisms are triggered in plants. Investigations conducted by the authors along with a literature review showed that in the case of abiotic factors the mechanism is based mainly on enzymatic intracellular reactions. In turn, non-enzymatic mechanisms are induced in the case of biotic stressors. However, earlier studies showed that this division is not absolutely clear cut. For this reason, the authors suggest that both these mechanisms in the case of the above-mentioned stress factors, particularly chemical protection, are triggered simultaneously, although to a different degree. An additional stress factor inducing biosynthesis of phenolic acids is connected with variable atmospheric conditions. In this study, the field experiment was conducted in a 3-year cycle.
The aim of this study was to determine changes in the quantitative profile of 12 phenolic acids, total bound phenolic acids, total free phenolic acids and antioxidant activity in grain of 23 winter wheat genotypes exposed to stress during cultivation. Stress was caused in two ways: the first experimental variant consisted in the induction of ear blight by artificial inoculation with spores of fungi from the genus Fusarium, while in the other variant chemical protection was provided by spraying fungicides.
Materials and methods
Location
The field experiment conducted in the years 2011–2013 was established in Poland, at the Mikulice Breeding and Production Station belonging to Małopolska Hodowla Roślin 50°00′26.7"N, 22°26′25.2"E and 117 m a.s.l.
Plant material
Twenty-three genotypes of winter wheat (Triticum aestivum L.) were selected on the basis of their resistance to Fusarium [7]. A total of 13 wheat forms came from Polish plant breeding companies. Three lines of winter wheat (82/2011, 83/2011 and 91/2011) were selected out at the Department of Genetics and Plant Breeding, the Poznan University of Life Sciences. The other seven genotypes are cultivars and lines coming from six European countries (Germany, the Czech Republic, Austria, Holland, Hungary and Sweden), which are used in breeding of winter wheat as sources of resistance to Fusarium.
Field trial
The experimental designs were randomized blocks with 23 wheat genotypes in the years 2011–2013. The experiment had two factors: one was the variant of protection and the other was the wheat genotype. All wheat genotypes were sown onto 3 m2 plots in three replications in three different variants of protection:
-
1)
control—no chemical crop protection, natural infestation (K);
-
2)
no chemical crop protection, inoculation with fungi from the genus Fusarium (I);
-
3)
full chemical crop protection—fungicides: Duet Ultra 497 SC (epoxyconazole, thiophanate methyl) at a concentration of 0.6 l/ha, and Capalo 337.5 SE (fenpropimorph, epoxyconazole, metrafenone) at 2 l/ha (POCH).
The inoculum used in inoculation was produced from six isolates of Fusarium fungi exhibiting high pathogenicity, i.e. three isolates each obtained from F. graminearum and F. culmorum, with the isolates being preliminarily tested in field experiments. Spore concentration was established at approx. 106 ml. Next suspensions of all isolates were mixed.
Wheat heads at anthesis were sprayed with the spore suspension at a rate of 100 ml/m2. Inoculations were performed individually on each plot at the beginning of anthesis and repeated about 3 days later at full anthesis.
From all genotypes, bulk samples of 200 g were collected at harvest, from which a sample of 100 g grain each was used in further analyses.
Analytical methods
Analysis of type A and B trichothecenes
Grain samples were analysed for the presence of trichothecenes according to Stuper-Szablewska and Perkowski [6]. The type A trichothecenes (H-2 toxin, T-2 toxin, T-2 tetraol) were analysed as TFAA derivatives. The type B trichothecenes (DON, NIV, 3-AcDON, 15-AcDON) were analysed as TMS (trimethylsilylsilyl ethers) derivatives. The analyses were run on a gas chromatograph (Hewlett Packard GC 6890) hyphenated to a mass spectrometer (Hewlett Packard 5972 A, Waldbronn, Germany) using an HP-5MS, 0.25 mm × 30 m capillary column.
Analysis of ergosterol
Ergosterol was determined by UPLC as described by Stuper-Szablewska and Perkowski [6] with some modifications. The samples were analysed by Aquity H class UPLC system equipped with a Waters Acquity PDA detector (Waters, USA). Chromatographic separation was performed on a Acquity UPLC® BEH C18 column (100mm × 2.1 mm,particle size 1.7 µm) (Waters, Ireland) and eluted with methanol/acetonitrile/water (85:10:5) at a flow rate of 0.4 ml/min. Ergosterol was detected with a Waters Acquity PDA detector (Waters, USA) set at 282 nm.
Analysis of free phenolic acids
Analysis of phenolic acids. Contents of 12 phenolic acids were determined as described by Stuper-Szablewska et al. [7].
Analysis of bound phenolic acids
Analysis of bound phenolic acids was made after alkaline hydrolysis and acid hydrolysis as described in Stuper-Szablewska et al. [8]. Analysis was performed using an Aquity H class UPLC system equipped with an Waters Acquity PDA detector (Waters, USA). Chromatographic separation was performed on a Acquity UPLC® BEH C18 column (100 mm × 2.1 mm,particle size 1.7 µm) (Waters, Ireland). A gradient elution was used, following mobile phase composition: A: acetonitryl with 0.1% formic acid, B: 1% aqueous formic acid mixture (pH = 2). Concentrations of phenolic compounds were determined using an internal standard at wavelengths λ = 320 nm and 280 nm.
Total antioxidant capacity
The automated method to measure ABTS, i.e. “antioxidant power”, was modified by Stuper-Szablewska et al. [8].
Statistical analysis
Results recorded in the course of the conducted chemical analyses were subjected to statistical analysis with the use of STATISTICA ver. 8.0 software. To compare the contents of individual metabolites in samples, Tukey’s multiple comparison procedure was used. Moreover, a stepwise linear discriminatory analysis (SLDA) was performed to separate groups in the analysed populations.
Results and discussion
The experiments on 23 winter wheat genotypes exhibiting varying resistance [8] were conducted in two aspects. One consisted in analyses of chemical parameters to determine the extent of fungal infection. The other line of experiments comprised analyses of bioactive compounds constituting the first line of plant response to the action of a stressor. The experiments were conducted in a 3-year cycle.
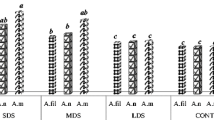
In the first stage, the degree of infestation and the level of mycotoxin contamination in the grain were verified. Wheat was grown in three experimental variants: the control (K), inoculation (I) and full chemical protection (POCH). Experimental conditions were described in “Materials and Methods”. Thus, the collected grain was analysed chemically to determine the level of mycotoxin contamination with type B trichothecenes. Statistically significant differences were found between the concentrations of all analysed mycotoxins for the inoculated variant, while no differences were observed in the control variants and those subjected to chemical protection (Table 1). At the same time, recorded results showed that inoculation with Fusarium fungi was progressing in a typical manner, resulting in a significant increase in trichothecene concentrations in wheat grain. This trend was repeated in a 3-year cycle. A significantly higher mycotoxin level was recorded in samples of inoculated grain in 2011. This was caused by the weather conditions promoting the development of late ear blight.
The mean annual precipitation levels ranged from 441 mm in 2012 to 595 mm in 2011. Climate data referring to the productive period are shown in Fig. 1. The period from April to July was characterized by high precipitation total in 2011 (415 mm), drought in 2012 (189 mm) and optimal precipitation total in 2013 (235 mm). In all the years of the study, temperatures in the period from April to July were comparable.
Concentrations of ergosterol (ERG) presented in Table 1 also confirm significantly higher levels of contamination with microscopic fungi in inoculated wheat samples in comparison to the control and chemically protected samples. In the case of this chemical marker for the infestation level, statistically significant differences were also observed between chemically protected and control samples. ERG is a marker both for dead and live mycelium; thus, these results indicate that fungal infection may have been developing prior to fungicide application and the administration of chemical protection inhibited the development of infection and biosynthesis of mycotoxins. When comparing the results between the years of the study 2011 was found to differ significantly from the other 2 years in terms of contamination with microscopic fungi. Knowing the degree of infestation and mycotoxin contamination, the levels of selected bioactive compounds and antioxidant activity were analysed.
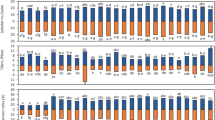
Literature data and earlier studies indicated a significant share of phenolic acids in the non-enzymatic resistance mechanism in plants exposed to biotic stressors. To date, it has been postulated that antioxidative reactions are activated in the case of plant infection [9,10,11,12,13]. Concentrations of bound and free phenolic acids were analysed (Fig. 2a, b). Comparable trends were observed in each year of the experiments. The highest concentrations of bound phenolic acids were recorded in inoculated samples. The control and chemically protected samples did not differ significantly. In 2011, contents of bound phenolic acids were 1.5-fold higher in inoculated samples in comparison to K and POCH samples. In the following years, that difference was on average 2.3-fold. The trends for the concentrations of free phenolic acids analysed next were different (Fig. 2b). In 2011, the highest concentrations were recorded in inoculated and chemically protected samples with no significant differences found between them. In the control samples, the amounts of free phenolic acids assayed in the grain were two times lower. In the years 2012–2013, significant differences were observed in terms of contents of free phenolic acids in grain between all the three experimental variants. However, this trend was similar to that for bound phenolic acids, i.e. inoculated samples contained the greatest amounts of free phenolic acids.
The next stage in the study was to determine antioxidant activity of wheat grain extracts coming from the three investigated experimental variants. The results (Fig. 2c) showed similar trends comparable to those for bound phenolic acids. In the case of K and POCH samples, they showed significantly higher activities than extracts from I samples, while additionally the two former did not differ statistically.
Finally, the chromatographic analysis of contents of 12 bound phenolic acids was carried out. As it was shown earlier [8], these acids are essential for resistance responses of wheat. It was not only ferulic acid, as it had been postulated before, but also the synergistic effect of ferulic, synapic, t-cinnamic and 4-hydroxybenzoic acids. Based on the results (Table 2), significant differences were found for contents of these acids in all the three experimental variants. The other analysed acids showed no quantitative differences between the K, I and POCH samples (Tables 2, 3). Variant I showed on average a fourfold greater content of ferulic acid in comparison to K samples. In the case of POCH samples, this difference was on average twofold.
The experimental variants have been based for comprised statistical analysis of the results in order to identify among the investigated chemical parameters the one exhibiting the greatest discriminatory power. The determined parameters should provide the best division of the investigated population into the experimental variants. Summing up, the factor of greatest importance during both biotic and abiotic stress was searched for. Stepwise discriminatory analysis was conducted (Fig. 3). The first stage of the analysis concerned parameters connected with fungal infection (Fig. 3a). The population of inoculated samples was significantly separated from the joint set of the control and POCH samples. The second variant included (Fig. 3b) 12 phenolic acids, total free and bound phenolic acids. The resulting separation was significantly greater than the previous one, but it was unsatisfactory, as no complete separation of the control and POCH samples was obtained. Nevertheless, statistical analysis showed that the sum of free phenolic acids and ferulic, synapic, t-cinnamic and 4-hydroxybenzoic acids exhibits the greatest discriminatory power. Only the third SLDA analysis based on the contents of selected bound phenolic acids (Fig. 3c), antioxidant activity and free phenolic acids led to a complete separation of the investigated populations of the experimental groups.
Conclusion
The statistical analysis confirmed earlier observations of the authors based on the results of chemical analyses, indicating that free phenolic acids are the most significant factors in resistance mechanisms as the first line of defence together with their antioxidant action, followed by the synergistic effect of exogenous bound phenolic acids: ferulic, synapic, t-cinnamic and 4-hydroxybenzoic acids (Fig. 4). Hypotheses presented in earlier literature sources concerning plant defence mechanisms were verified based on this study and supplemented with the data presented here. The antioxidative mechanism serves a significant role during plant exposure both to biotic and abiotic stressors. However, it is only one element of the plant defence system against stress. Bound phenolic acids serve other functions in plant cells [14] and so far their antifungal action has not been fully clarified. Free phenolic acids play a significant role in plant resistance mechanisms irrespective of the type of stress factors.
References
Ashraf M, Harris PJC (2005) Abiotic stresses plant resistance through breeding and molecular approaches. Food Products Press, New York, pp 1–725
Liu WZ, Langseth W, Skinnes H, Elen ON, Sundheim L (1997) Comparison of visual head blight ratings, seed infection levels, and deoxynivalenol production for assessment of resistance in cereals inoculated with Fusarium culmorum. Eur J Plant Pathol 103:589–595
Langevin F, Eudes F, Comeau A (2004) Effect of trichothecenes produced by Fusarium graminearum during Fusarium head blight development in six cereal species. E J Plant Pathol 110:735–746
Bottalico A (1998) Fusarium diseases of cereals: species complex and related mycotoxin profiles, in Europe. J Plant Pathol 80:85–103
Miedaner T, Wilde F, Korzun V, Ebmeyer E, Schmolke M, Hartl L et al (2009) Marker selection for Fusarium head blight resistance based on quantitative trait loci (QTL) from two European sources compared to phenotypic selection in winter wheat. Euphytica 166:219–227
Stuper-Szablewska K, Perkowski J (2017) Level of contamination with mycobiota and contents of mycotoxins from the group of trichotchecenes in grain of wheat, oat, barley, rye and triticale harvested in Poland in the years of 2006–2008. Ann Agric Environ Med 24(1):49–556
Stuper-Szablewska K, Kurasiak-Popowska D, Nawracała J, Perkowski J (2014) Comparison of phenolic acid contents in various wheat genotypes. Przem Chem 93(12):2274–2278
Stuper-Szablewska K, Kurasiak-Popowska D, Nawracała J, Perkowski J (2017) Response of non enzymatic antioxidative mechanisms to stress caused by infection with Fusarium fungi and chemical protection in different wheat genotypes. Chem Ecol 33(10):949–962
Działo M, Mierziak J, Korzun U, Preisner M, Szopa J, Kulma A (2016) The potential of plant phenolics in prevention and therapy of skin disorders. Int J Mol Sci 17(2):160
Gupta VK, Sharma SK (2006) Plants as natural antioxidants. Nat Prod Rad 5(4):326–334
Kasote DM, Katyare SS, Hegde MV, Bae H (2015) Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci 11(8):982–991
Kulbat K (2016) The role of phenolic compounds in plant resistance. Biotechnol Food Sci 80:97–108
Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X, Kong M, Li L, Zhang Q, Liu Y (2016) An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 21:1374
Mandal SM, Chakraborty D, Dey S (2010) Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav 5(4):359–368
Acknowledgements
The authors are grateful for the financial support provided by The Ministry of Agriculture and Rural Development (Basic research for biological progress in crop production: HOR hn 801-13/12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflicts of interest exist in the sub-mission of this manuscript.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Stuper-Szablewska, K., Kurasiak-Popowska, D., Nawracała, J. et al. Quantitative profile of phenolic acids and antioxidant activity of wheat grain exposed to stress. Eur Food Res Technol 245, 1595–1603 (2019). https://doi.org/10.1007/s00217-019-03262-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03262-8