Abstract
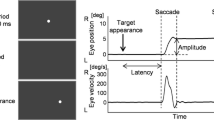
Parkinson’s disease (PD) is associated with a loss of central dopaminergic pathways in the brain leading to an abnormality of movement, including saccades. In PD, analysis of saccadic latency distributions, rather than mean latencies, can provide much more information about how the neural decision process that precedes movement is affected by disease or medication. Subject to the constraints of intersubject variation and reproducibility, latency distribution may represent an attractive potential biomarker of PD. Here we report two studies that provide information about these parameters, and demonstrate a novel effect of dopamine on saccadic latency, implying that it influences the neural decision process itself. We performed a detailed cross-sectional study of saccadic latency distributions during a simple step task in 22 medicated patients and 27 age-matched controls. This revealed high intersubject variability and an overlap of PD and control distributions. A second study was undertaken on a different population specifically to investigate the effects of dopamine on saccadic latency distributions in 15 PD patients. l-dopa was found to prolong latency, although the magnitude of the effect varied between subjects. The implications of these observations for the use of saccadic latency distributions as a potential biomarker of PD are discussed, as are the effects of l-dopa on neural decision making, where it is postulated to increase the criterion level of evidence required before the decision to move is made.





Similar content being viewed by others
References
Braun D, Weber H, Mergner T, Schulte-Monting J (1992) Saccadic reaction times in patients with frontal and parietal lesions. Brain 115(Pt 5):1359–1386
Briand KA, Strallow D, Hening W, Poizner H, Sereno AB (1999) Control of voluntary and reflexive saccades in Parkinson’s disease. Exp Brain Res 129:38–48
Carpenter RHS (1981) Oculomotor procrastination. In: Fisher DF, Monty RA, Senders JW (eds) Eye movements: cognition and visual perception. Lawrence Erlbaum, New Jersey, pp 237–246
Carpenter RHS (1988) Movements of the eyes. Pion, London
Carpenter RHS (1994) SPIC: a PC-based system for rapid measurement of saccadic responses. J Physiol (Proceedings) 480:4P
Carpenter RHS (1999) A neural mechanism that randomises behaviour. Journal of Consciousness Studies 6(1):13–22
Carpenter RHS (2000) The neural control of looking. Curr Biol 10:R291–R293
Carpenter RHS, Williams ML (1995) Neural computation of log likelihood in control of saccadic eye movements. Nature 377:59–62
Cools R, Barker RA, Sahakian BJ, Robbins TW (2001) Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex 11:1136–1143
Cools R, Barker RA, Sahakian BJ, Robbins TW (2003) L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia 41:1431–1441
Crevits L, Versijpt J, Hanse M, De Ridder K (2000) Antisaccadic effects of a dopamine agonist as add-on therapy in advanced Parkinson’s patients. Neuropsychobiology 42:202–206
Djamgoz MB, Hankins MW, Hirano J, Archer SN (1997) Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision Res 37:3509–3529
Dursun SM, Wright N, Reveley MA (1999) Effects of amphetamine on saccadic eye movements in man: possible relevance to schizophrenia? J Psychopharmacol 13:245–247
Fahn S, Elton RL, and committee (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Caine D, Goldstein M (eds) Recent developments in Parkinson’s disease. Florham Park, NJ: McMillan
Foltynie T, Brayne CE, Robbins TW, Barker RA (2004a) The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain 127:550–560
Foltynie T, Goldberg TE, Lewis SG, Blackwell AD, Kolachana BS, Weinberger DR, Robbins TW, Barker RA (2004b) Planning ability in Parkinson’s disease is influenced by the COMT val158met polymorphism. Mov Disord 19:885–891
Foltynie T, Lewis SG, Goldberg TE, Blackwell AD, Kolachana BS, Weinberger DR, Robbins TW, Barker RA (2005) The BDNF Val(66)Met polymorphism has a gender specific influence on planning ability in Parkinson’s disease. J Neurol
Gauntlett-Gilbert J, Brown VJ (1998) Reaction time deficits and Parkinson’s disease. Neurosci Biobehav Rev 22:865–881
Gaymard B, Lynch J, Ploner CJ, Condy C, Rivaud-Pechoux S (2003) The parieto-collicular pathway: anatomical location and contribution to saccade generation. Eur J Neurosci 17:1518–1526
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Gibson JM, Kennard C (1987) Quantitative study of “on-off” fluctuations in the ocular motor system in Parkinson’s disease. Adv Neurol 45:329–333
Gibson JM, Pimlott R, Kennard C (1987) Ocular motor and manual tracking in Parkinson’s disease and the effect of treatment. J Neurol Neurosurg Psychiatry 50:853–860
Gordon PH, Yu Q, Qualls C, Winfield H, Dillon S, Greene PE, Fahn S, Breeze RE, Freed CR, Pullman SL (2004) Reaction time and movement time after embryonic cell implantation in Parkinson disease. Arch Neurol 61:858–861
Gotham AM, Brown RG, Marsden CD (1988) ‘Frontal’ cognitive function in patients with Parkinson’s disease ‘on’ and ‘off’ levodopa. Brain 111(Pt 2):299–321
Hanes DP, Schall JD (1996) Neural control of voluntary movement initiation. Science 274:427–430
Harrison J, Henderson L, Kennard C (1995) Abnormal refractoriness in patients with Parkinson’s disease after brief withdrawal of levodopa treatment. J Neurol Neurosurg Psychiatry 59:499–506
Hikosaka O, Takikawa Y, Kawagoe R (2000) Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80:953–978
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Horwitz GD, Batista AP, Newsome WT (2004) Representation of an abstract perceptual decision in macaque superior colliculus. J Neurophysiol 91:2281–2296
Hughes AJ, Ben Shlomo Y, Daniel SE, Lees AJ (1992) What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology 42:1142–1146
Hughes AJ, Daniel SE, Ben Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125:861–870
Kim JN, Shadlen MN (1999) Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci 2:176–185
Kori A, Miyashita N, Kato M, Hikosaka O, Usui S, Matsumura M (1995) Eye movements in monkeys with local dopamine depletion in the caudate nucleus. II. Deficits in voluntary saccades. J Neurosci 15:928–941
Leigh RJ, Kennard C (2004) Using saccades as a research tool in the clinical neurosciences. Brain 127:460–477
Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA (2005) Heterogeneity of Parkinson’s disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry 76:343–348
Lynch G, King DJ, Green JF, Byth W, Wilson-Davis K (1997) The effects of haloperidol on visual search, eye movements and psychomotor performance. Psychopharmacology (Berl) 133:233–239
McCoy AN, Platt ML (2005) Expectations and outcomes: decision-making in the primate brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191:201–211
Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF (2005) Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci 8:594–596
Micallef-Roll J, Rihet P, Hasbroucq T, Possamai C, Blin O (2001) Levodopa-induced drowsiness in healthy volunteers: results of a choice reaction time test combined with a subjective evaluation of sedation. Clin Neuropharmacol 24:91–94
Michell AW, Lewis SJ, Foltynie T, Barker RA (2004) Biomarkers and Parkinson’s disease. Brain 127:1693–1705
Mosimann UP, Muri RM, Burn DJ, Felblinger J, O’Brien JT, McKeith IG (2005) Saccadic eye movement changes in Parkinson’s disease dementia and dementia with Lewy bodies. Brain 128:1267–1276
Muller T, Benz S, Przuntek H (2002) Apomorphine delays simple reaction time in Parkinsonian patients. Parkinsonism Relat Disord 8:357–360
Ober JK, Przedpelska-Ober E, Gryncewicz W, Dylak J, Carpenter RS, Ober JJ (2003) Hand-held system for ambulatory measurement of saccadic durations of neurological patients. In: Gadja J (eds) Modelling and Measurement in Medicine. Komitet Biocybernityki i Inzyneierii Biomedycznej. PAN, Warsaw pp 187–198
Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y (1991) Cortical control of reflexive visually-guided saccades. Brain 114(Pt 3):1473–1485
Rascol O, Clanet M, Montastruc JL, Simonetta M, Soulier-Esteve MJ, Doyon B, Rascol A (1989) Abnormal ocular movements in Parkinson’s disease. Evidence for involvement of dopaminergic systems. Brain 112(Pt 5):1193–1214
Reddi BA, Carpenter RH (2000) The influence of urgency on decision time. Nat Neurosci 3:827–830
Reddi BA, Asrress KN, Carpenter RH (2003) Accuracy, information, and response time in a saccadic decision task. J Neurophysiol 90:3538–3546
Robbins TW, Brown VJ (1990) The role of the striatum in the mental chronometry of action: a theoretical review. Reviews in the Neurosciences 2:181–213
Romo R, Schultz W (1987) Neuronal activity preceding self-initiated or externally timed arm movements in area 6 of monkey cortex. Exp Brain Res 67:656–662
Romo R, Schultz W (1992) Role of primate basal ganglia and frontal cortex in the internal generation of movements. III. Neuronal activity in the supplementary motor area. Exp Brain Res 91:396–407
Roy-Byrne P, Radant A, Wingerson D, Cowley DS (1995) Human oculomotor function: reliability and diurnal variation. Biol Psychiatry 38:92–97
Schall JD (1999) Weighing the evidence: how the brain makes a decision. Nat Neurosci 2:108–109
Shadlen MN, Newsome WT (1996) Motion perception: seeing and deciding. Proc Natl Acad Sci U S A 93:628–633
Stocchi F, Olanow CW (2003) Neuroprotection in Parkinson’s disease: clinical trials. Ann Neurol 53(Suppl 3):S87–S97
Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW (2000) Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia 38:596–612
Vermersch AI, Rivaud S, Vidailhet M, Bonnet AM, Gaymard B, Agid Y, Pierrot-Deseilligny C (1994) Sequences of memory-guided saccades in Parkinson’s disease. Ann Neurol 35:487–490
Wang M, Vijayraghavan S, Goldman-Rakic PS (2004) Selective D2 receptor actions on the functional circuitry of working memory. Science 303:853–856
Wilcox RE, Spirduso WW (1988) Apomorphine doses impair the reaction time of fast reacting but not slow reacting rats. Psychopharmacology (Berl) 95:276–279
Williams GV, Goldman-Rakic PS (1995) Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376:572–575
Acknowledgements
These studies have been funded by an MRC COGG grant into PD and its heterogeneity, and the BCNC. AWM holds a PDS studentship, and a Raymond and Beverly Sackler Award. We thank Dr A.Blackwell for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
RHS Carpenter and RA Barker are joint senior authors for this paper
Rights and permissions
About this article
Cite this article
Michell, A.W., Xu, Z., Fritz, D. et al. Saccadic latency distributions in Parkinson’s disease and the effects of l-dopa. Exp Brain Res 174, 7–18 (2006). https://doi.org/10.1007/s00221-006-0412-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0412-z