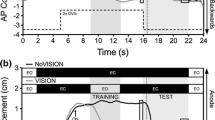
Abstract
The perception of angular displacement during self turning is generally based on a combination of redundant signals from different sources. For example, during active turning in a visually structured environment devoid of landmarks, podokinesthetic, vestibular, and optokinetic velocity signals are fused and integrated over time to yield a unitary percept of the ongoing change in angular position (‘podokinesthetic’ refers to proprioceptive and corollary signals related to leg and foot movement). Previously we have shown that the fusion of two of these afferents improves perceptual accuracy and reliability in comparison to when only one is available. For example, with only a single modality available, slow rotations are perceived to be significantly larger than fast ones, whereas the combination of two modalities greatly reduces this difference. These observations spurred the hypothesis that displacement perception results from a weighted average of bottom-up (sensory) signals and top-down signals (a priori knowledge or expectation), with the weight of the latter decreasing the more sensory information is available. We now ask (1) whether the accuracy of angular displacement estimation can be further improved if it can draw on all three sensory modalities instead of only two, and (2) whether bottom-up sensory and top-down a priori information is combined for displacement estimation in a statistically optimal way. To this end 12 healthy subjects (Ss) standing on a turning platform surrounded by a rotatable optokinetic pattern were exposed to 6 different sensory conditions: pure podokinesthetic (P), vestibular (V), or optokinetic (O) stimulation, and combined podokinesthetic-vestibular (PV), vestibular-optokinetic (VO), or podokinesthetic-vestibular-optokinetic (PVO) stimulation. Stimuli had constant angular velocities of either 15, 30, or 60°/s. Subjects were to press a signal button when they felt that angular displacement had reached a previously instructed magnitude (150–900°). In agreement with earlier observations, the combination of two sensory signals improved the accuracy of displacement perception by reducing both the variance of subjects’ displacement estimates and their dependence on turning velocity. Adding a third sensory signal (condition PVO) led to a further reduction of variance and almost eliminated the effect of velocity. We show that these experimental results are compatible with a probabilistic fusion mechanism based on Bayes’ law. This mechanism would operate on logarithmic representations of turning velocity and proceed in two stages. A first stage fuses all available bottom-up information to create a unitary representation of the velocity signalled by the different sensory modalities. A second stage then fuses this sensory information with top-down a priori information; the latter creates a bias in favour of a ‘default velocity’ that grows as the uncertainty of the sensory information increases. Our experimental data agree with the relation between (1) the variance of displacement estimates and (2) their modulation by velocity predicted by this scheme.





Similar content being viewed by others
Notes
Gain curves from conditions P and V have already been presented in (Becker et al. 2002).
In previous work where we have observed a similar dependence of gain on turning velocity we had defined a ‘percentage velocity spread index’, VSI% = [GMI(30/15)·GMI(60/30)−1]/3 × 100%, instead (Jürgens et al. 2003).
References
Anastasio TJ, Patton PE, Belkacem-Boussaid K (2000) Using Bayes’ rule to model multisensory enhancement in the superior colliculus. Neural Comput 12:1165–1187
Bakker NH, Werkhoven PJ, Passenier PO (1999) The effects of proprioceptive and visual feedback on geographical orientation in virtual environments. Presence 8:36–53
Battaglia PW, Jacobs RA, Aslin RN (2003) Bayesian integration of visual and auditory signals for spatial localization. J Opt Soc Am [A] 20:1391–1397
Becker W, Nasios G, Raab S, Jürgens R (2002) Fusion of vestibular and podokinesthetic information during self-turning towards instructed targets. Exp Brain Res 144:458–474
van Beers R, Sittig AC, Denier van der Gon JJ (1999) Integration of proprioceptive and visual position-information: an experimentally supported model. J Neurophysiol 81:1355–1364
Brandt T, Glasauer S, Stephan T, Bense S, Yousry TA, Deutschländer A, Dieterich M (2002) Visual-vestibular and visuovisual cortical interactions. Ann NY Acad Sci 956:230–241
Deneve S, Pouget A (2004) Bayesian multisensory integration and cross-modal spatial links. J Physiol Paris 98:249–258
Ernst MO, Banks MS (2002) Humans integrate visual and haptic information in a statistically optimal fashion. Nature (London) 415:429–433
Guo K, Nevado A, Robertson RG, Pulgarin M, Thiele A, Young MP (2004) Effects on orientation perception of manipulating the spatio-temporal prior probability of stimuli. Vision Res 44:2349–2358
Hürlimann F, Kiper DC, Carandini M (2002) Testing the Bayesian model of perceived speed. Vision Res 42:2253–2257
Howard IP (1997) Interactions within and between the spatial senses. J Vestib Res 7:311–345
Jürgens R, Nasios G, Becker W (2003) Vestibular, optokinetic, and cognitive contribution to the guidance of passive self-rotation toward instructed targets. Exp Brain Res 151:90–107
Jacobs RA (1999) Optimal integration of texture and motion cues to depth. Vision Res 39:3621–3629
Knill DC, Pouget A (2004) The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci 27:712–719
Knill DC, Saunders JA (2003) Do humans optimally integrate stereo and texture information for judgements of surface slant? Vision Res 43:2539–2558
Körding KP, Wolpert DM (2004) Bayesian integration in sensorimotor learning. Nature 427:244–247
Marlinsky VV (1999) Vestibular and vestibulo-proprioceptive perception of motion in the horizontal plane in blindfolded man - II. Estimations of rotations about the earth-vertical axis. Neuroscience 90:395–401
Maunsell JH, Van Essen DC (1983) Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation 49:1127–1147
Mergner T, Siebold C, Schweigart G, Becker W (1991) Human perception of horizontal trunk and head rotation in space during vestibular and neck stimulation. Exp Brain Res 85:389–404
Mergner T, Schweigart G, Müller M, Hlavacka F, Becker W (2000) Visual contributions to human self-motion perception during horizontal body rotation. Arch Ital Biol 138:139–166
Nover H, Anderson CH, DeAngelis GC (2005) A logarithmic, scale-invariant representation of speed in macaque middle temporal area accounts for speed discrimination performance. J Neurosci 25:10049–10060
Papoulis A (1965) Probability, random variables and stochastic processes. McGraw-Hill, NewYork
Poulton EC (1977) Quantitative subjective assessments are almost always biased, sometimes completely misleading. Br J Psychol 68:409–425
Stocker AA, Simoncelli EP (2005) Constraining a Bayesian model of human visual speed perception. In: Saul K, Weiss Y, Bottou L (eds) Advances in neural information proceeding systems NIPS 17:1361–1368
Taube JS (1995) Head-direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci 15:70–86
Weiss Y, Simoncelli EP, Adelson EH (2002) Motion illusions as optimal percepts. Nat Neurosci 5:598–604
Young LR (1981) Perception of the body in space: mechanisms. In: Geiger SR (ed) Handbook of physiology, Section 1: The nervous system, Vol III. American Physiological Society, Bethesda, pp 1023–1066
Acknowledgements
We are indebted to Ralph Kühne for his support with electronic and data processing equipment and to Bruno Glinkemann for taking care for the mechanical equipment. This work was supported by Deutsche Forschungsgemeinschaft, grant Be 783/3.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
We here give supplementary details regarding the assumptions and calculations underlying the Bayesian interpretation paragraph in the Discussion section.
Symbols and assumptions
Referring to Fig. 4, we will use the following symbols:
General
Overlined symbols (e.g., \(\bar v_{\rm p})\) denote the mean (experimental data) or central value (probability distributions) of variables. Assumptions are being referred by square brackets [].
Signals and parameters in linear space
- v 0 :
-
Default velocity or ‘prior’
- v i(i=1–3):
-
Afferent signals from sensors P, V, O, respectively
- v st :
-
Stimulus velocity
- v s :
-
Compound sensory signal corresponding to ψs
- v p :
-
‘Perceived’ velocity (internal estimation of stimulus velocity)
- C :
-
Calibration factor
- D p :
-
Perceived angular displacement
- D A, D D :
-
Achieved and desired displacements as defined in Methods
- G T :
-
Targeting gain as defined in Methods
- GMI :
-
Gain modulation index as defined in Results
- t :
-
Time from start of trial
Signals and parameters in logarithmic space
- ψ i (i=0–3):
-
Log-transforms of v i
- ψs :
-
Compound sensory signal; identical to ψi when only a single channel i is active (monomodal case); result of fusing several signals in case of multimodal stimulation
- ψf :
-
Result of fusing prior and compound sensory signal
- ψp :
-
Log-transform of v p
- σ 20 , σ 2s :
-
Variances of ψ0 and ψs
- σ 2f , σ 2p :
-
Variances of ψf and ψp
- σ 2r :
-
Residual variance
- w 0, w s :
-
Relative weights of, respectively, prior and compound sensory signal during fusion
All numeric values to be reported for these variables are based on the natural logarithm.
Assumptions
-
[A1]
v i (i = 1, 2, 3) are statistically independent, noisy signals with approximately normal (Gaussian) distribution all having central values equal to v st (‘concordant’ information) throughout a run and ‘sufficiently’ (see below) small variance.
-
[A2]
ψ0 has normal distribution; its central value (the log equivalent of v 0) remains constant throughout a run and is unrelated to the actual magnitude of v st and, by the same token, to that of v i (i = 1, 2, 3).
-
[A3]
The residual noise σ 2r has normal distribution.
-
[A4]
ψp has ‘sufficiently’ small variance.
We call the variances σ2(v) or σ2(ψ) of, respectively, normally distributed variables v or ψ ‘sufficiently’ small, if their transforms log{v} or log−1{ψ} also have an approximately normal distribution. The following approximations then hold; \(\overline {{1 \mathord{\left/ {\vphantom {1 v}} \right. \kern-\nulldelimiterspace} v}} \approx {1 \mathord{\left/ {\vphantom {1 {\bar v}}} \right. \kern-\nulldelimiterspace} {\bar v}}, \sigma (v/\bar v) \approx \sigma (\bar v/v), \sigma (\log \{ v\}) \approx {{\sigma (v)} \mathord{\left/ {\vphantom {{\sigma (v)} {\bar v}}} \right. \kern-\nulldelimiterspace} {\bar v}},\) and likewise for ψ. Most variables to be considered below have standard deviations σ of less than 25% of their mean. Although this creates large deviations at the low and high ends of the distribution of their inverse, the standard deviation of the inverse differs by less than 5% from that of the original variable. We therefore will treat all of the above variables (except obviously v st) as Gaussians.
Basic relations
Subjects were supposed to deliver their ‘on-target’-signal when D p = C·v p·t reached the desired value D D; given [A1] and because D A = v st·t the following relation between targeting gain and perceived velocity results:
hence
Noting that log(v st/C) = constant and log(v p) ≡ ψp, we therefore obtain
that is, the variance of the perceived velocity’s logarithmic representation can be estimated from the experimentally observed values of the targeting gain.
Bayesian interaction is unlikely to take place in linear space
An interaction between prior and sensory signals on a linear rather than a log-scale cannot explain the experimentally observed characteristics of GMI. As noted in Results, the standard deviation of v p rises in proportion to v st from whence it can be inferred that also that of v s would be a rising function of v st. Therefore, if signals v 0 and v s rather than their log representations ψ0 and ψs were combined according to Eqs. 2 and 3, prior weight w 0 would be much larger with v st = 60°/s than with 15°/s and, consequently, GMI(60/30) would be larger than GMI(30/15). Using reasonable first order approximations for v 0 (30°/s) and w 0 (0.2), this difference can be estimated to be in the order of 25%. However, the experimentally observed GMI(60/30) did not significantly differ from GMI(30/15) being either slightly smaller (4 conditions) or equal (2 conditions), but definitely not larger (Fig. 2). Hence, a Bayesian fusion in linear space does not yield an appropriate description of our data.
Baysian interaction predicts a lawful relation between GMI and the variance of G T
According to Fig. 4
Because the addition of residual noise σ 2r does not change the central value, Eq. 2 (cf. Discussion) implies
or, expressed in terms of the linearly coding signals v p, v 0 and v s, given [A1]:
Let \(\bar v_{\rm s1} \) and \(\bar v_{\rm s2} \) be the mean sensory velocity signals evoked by v st1 and v st2 = 2·v st1 (e.g. 15 and 30°/s), and \(\bar G_{\rm T1} \) and \(\bar G_{\rm T2} \) the corresponding mean targeting gains. Combining Eqs. 5 and 8 and noting that \(v_{\rm st}=\bar v_{\rm s}[\text{A1}]w\) we obtain
Because w s=1−w 0, GMI can also be expressed in terms of w 0:
or, because [A1] implies \(\bar v_{\rm s2}/\bar v_{\rm s1} = 2,\)
Combining Eqs. 3 and 4 and taking into account the residual variance by noting σ 2f = σ 2p − σ 2r (Fig. 4) w 0 can be written as
hence Eq. 9 becomes
and, finally, solving for σ 2p we obtain
Equation 11 predicts a linear relationship between variance σ 2p and the log transform of GMI. Because both σ 2p and log{GMI} can be obtained from observed data, we can use Eq. 11 to check the compatibility of our data with the Bayesian hypothesis and estimate the unknown parameters σ 20 and σ 2r by linear regression (Fig. 5). Because the estimate for the standard deviation of ψ0 obtained in this way (σ0 = 0.4) is not sufficiently small in the sense of [A1], its standard deviation in linear space was approximated by fitting the log−1-transform of a Gaussian with σ = 0.4 by a new Gaussian (this does not touch the validity of the above equations as none requires ψ0 to have small variance).
Based on the estimated values of σ 20 and σ 2r and on Eq. 4 the variance (σ 2s ) of the compound sensory signal can be obtained from
An expression for the mean default velocity \(\bar v_0 \) is obtained from Eq. 8:
which, using Eq. 5 becomes
Rights and permissions
About this article
Cite this article
Jürgens, R., Becker, W. Perception of angular displacement without landmarks: evidence for Bayesian fusion of vestibular, optokinetic, podokinesthetic, and cognitive information. Exp Brain Res 174, 528–543 (2006). https://doi.org/10.1007/s00221-006-0486-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0486-7