Abstract
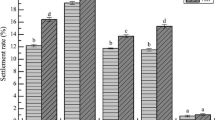
Larvae of many sessile marine invertebrates settle in response to surface microbial communities (biofilms), but the effects of soluble compounds from biofilms in affecting larval behavior prior to settlement, attachment, and metamorphosis have been little studied. This question was addressed by videotaping the behavior of competent larvae of the serpulid, Hydroides elegans, above settlement-inducing biofilms. Adult worms were collected in Pearl Harbor, Hawaii, USA in November 2012 and spawned almost immediately. Six-day old larvae were placed in five replicated treatments in small cups: (1) with a natural biofilm; (2) with a natural biofilm on an 8-µm screen, 1 mm above the bottom of a clean cup; (3) with a natural biofilm beneath a clean screen; (4) in a clean cup; and (5) in a clean cup with a clean screen. Using the videotapes, larval swimming speeds and trajectories were quantified within 5 min of the larvae being placed in a treatment. Only larvae that touched a biofilm, i.e., in treatments (1) and (2), slowed their swimming speed and increased the amount of time spent crawling rather than swimming. This shows that under these conditions, any soluble cues emanating from a biofilm do not affect settlement behavior. Furthermore, after 24 h close to 100 % of larva in the two accessible biofilm treatments had metamorphosed and <15 % in treatments that included a biofilm under a clean screen and no biofilm at all, strongly suggesting that soluble cues for settlement were not produced by the biofilms over the longer time period.








Similar content being viewed by others
References
Carpizo-Ituarte E, Hadfield MG (1998) Stimulation of metamorphosis in the polychaete Hydroides elegans Haswell (Serpulidae). Biol Bull 194:14–24
Crisp DJ (1974) Factors influencing the settlement of marine invertebrate larvae. In: Grant P, Mackie A (eds) Chemoreception in marine organisms. Academic Press, London, pp 177–265
Crisp DJ, Meadows PS (1963) Adsorbed layers: the stimulus to settlement in barnacles. Proc R Soc Lond B 158:364–387
Elbourne PD, Clare AS (2010) Ecological relevance of a conspecific, waterborne settlement cue in Balanus amphitrite (Cirripedia). J Exp Mar Biol Ecol 392:99–106
Elbourne PD, Veater RA, Clare AS (2008) Interaction of conspecific cues in Balanus amphitrite Darwin (Cirripedia) settlement assays: continued argument for the single-larva assay. Biofouling 24:87–96
Hadfield MG (2011) Biofilms and marine invertebrate larvae: what bacteria produce that larvae use to choose settlement sites. Ann Rev Mar Sci 3:453–479
Hadfield MG, Koehl MAR (2004) Rapid behavioral responses of an invertebrate larva to dissolved settlement cue. Biol Bull 207:28–43
Hadfield MG, Paul VJ (2001) Natural chemical cues for settlement and metamorphosis of marine invertebrate larvae. In: McClintock JB, Baker W (eds) Marine chemical ecology. CRC Press, pp 431–461
Hadfield MG, Pennington JT (1990) Nature of the metamorphic signal and its internal transduction in larvae of the nudibranch Phestilla sibogae. Bull Mar Sci 46:455–464
Hadfield MG, Unabia CC, Smith CM, Michael TM (1994) Settlement preferences of the ubiquitious fouler Hydroides elegans. In: Thompson MF, Nagabhushanam R, Sarojini R, Fingerman M (eds) Recent developments in biofouling control. Oxford and IBH Pub. Co., New Delhi, pp 65–74
Harder T, Qian P-Y (1999) Induction of larval attachment and metamorphosis in the serpulid polychaete Hydroides elegans by dissolved free amino acids: isolation and identification. Mar Ecol Prog Ser 179:259–271
Harder T, Lau S, Dahms H-U, Qian P-Y (2002) Isolation of bacterial metabolites as natural inducers for larval settlement in the marine polychaete Hydroides elegans (Haswell). J Chem Ecol 28:2029–2043
Herrmann K (1979) Larvallentwicklung und metamorphose von Phoronis psammophila (Phoronida, Tentaculata). Helgol Wiss Meeresunters 32:550–581
Herrmann K (1995) Induction and regulation of metamorphosis in the planktonic larvae: Phoronis mulleri (Tentaculata) as archetype. Helgol Meeresunters 49:255–281
Hidu H (1969) Gregarious setting in the American oyster Crassostrea virginica Gmelin. Chesap Sci 10:85–92
Huang S, Hadfield MG (2003) Composition and density of bacterial biofilms determine larval settlement of the polychaete Hydroides elegans. Mar Ecol Prog Ser 260:161–172
Huang Y, Callahan S, Hadfield MG (2012) Recruitment in the sea: bacterial genes required for inducing larval settlement in a marine worm. Sci Rep 2:228. doi:10.1038/srep00228
Kirchman D, Graham S, Reish D, Mitchell R (1982a) Lectins may mediate in the settlement and metamorphosis of Janua (Dexiospira) brasiliensis Grube (Polychaeta: Spirorbidae). Mar Biol Lett 3:131–142
Kirchman D, Graham S, Reish D, Mitchell R (1982b) Bacteria induce settlement and metamorphosis of Janua (Dexiospira) brasiliensis Grube (Polychaeta: Spirorbidae). J Exp Mar Biol Ecol 56:153–163
Koehl M, Hadfield M (2004) Soluble settlement cue in slowly moving water within coral reefs induces larval adhesion to surfaces. J Mar Syst 49:75–88
Koehl M, Strother J, Reidenbach M, Koseff J, Hadfield M (2007) Individual-based model of larval transport to coral reefs in turbulent, wave-driven flow: effects of behavioral responses to dissolved settlement cues. Mar Ecol Prog Ser 335:1–18
Koehl M, Crimaldi J, Dombroski D (2013) Wind chop and ship wakes determine hydrodynamic stresses on larvae settling on different microhabitats in fouling communities. Mar Ecol Prog Ser 479:47–62
Krug PJ, Zimmer RK (2000) Larval settlement: chemical markers for tracing production, transport, and distribution of a waterbourne cue. Mar Ecol Prog Ser 207:283–296
Lau SCK, Harder T, Qian P-Y (2003) Induction of larval settlement in the serpulid polychaete Hydroides elegans (Haswell): role of bacterial extracellular polymers. Biofouling 19:197–204
Lau SCK, Thiyagarajan V, Cheung CK, Qian P-Y (2005) Roles of bacterial community composition in biofilms as a mediator for larval settlement of three marine invertebrates. Aquat Microb Ecol 38:41–51
Leise EM, Froggett SJ, Nearhoof JE, Cahoon LB (2009) Diatom cultures exhibit differential effects on larval metamorphosis in the marine gastropod Ilyanassa obsoleta (Say). J Exp Mar Biol Ecol 379:51–59
Marechal JP, Hellio C, Sebire M, Clare AS (2004) Settlement behaviour of marine invertebrate larvae measured by EthoVision 3.0. Biofouling 20:211–217
Matson PG, Steffen BT, Allen RM (2010) Settlement behavior of cyphonautes larvae of the bryozoan Membranipora membranacea in response to two algal substrata. Invert Biol 129:277–283
Nedved BT, Hadfield MG (2009) Hydroides elegans (Annelida: Polychaeta): a model for biofouling research. In: Flemming H-C, Murthy PS, Venkatesan R, Cooksey K (eds) Marine and industrial biofouling. Springer, Berlin Heidelberg, pp 203–217
Okamoto K, Watanabe A, Sakata K, Watanabe N (1998) Chemical signals involved in larval metamorphosis in Hydroides ezoensis (Serpulidae; Polychaeta). Part I: induction of larval metamorphosis by extract of adult tube clumps. J Mar Biotech 6:7–10
Pawlik JR (1992) Induction of marine invertebrate larval settlement: evidence for chemical cues. In: Paul VJ (ed) Ecological roles of marine natural products. Cornell Univ. Press, Ithaca, pp 189–236
Penniman JR, Doll MK, Pires A (2013) Neural correlates of settlement in veliger larvae of the gastropod, Crepidula fornicata. Invertebr Biol 132:14–26
Reidenbach MA, Koseff JR, Monismith SG, Steinbuck JV, Genin A (2006) The effects of waves and morphology on mass transfer within branched reef corals. Limnol Oceanogr 51:1134–1141
Santagata S (2004) Larval development of Phoronis pallida (Phoronida): implications for morphological convergence and divergence among larval body plans. J Morphol 259:347–358
Scheltema RS (1961) Metamorphosis of the veliger larvae of Nassarius obsoletus (Gastropoda) in response to bottom sediment. Biol Bull 120:92–109
Scheltema RS, Williams IP, Shaw MA, Loudon C (1981) Gregarious settlement by the larvae of Hydroides dianthus (Polychaeta: Serpulidae). Mar Ecol Prog Ser 5:69–74
Shikuma NJ, Pilhofer M, Weiss GL, Hadfield MG, Jensen GJ, Newman DK (2014) Marine tubeworm metamorphosis induced by arrays of bacterial phage tail-like structures. Science 343:529–533
Sokal RR, Rohlf FJ (1981) Biometry. W.H. Freeman & Co., New York
Swanson RL, de Nys R, Huggett MJ, Green JK, Steinberg PD (2006) In situ quantification of a natural settlement cue and recruitment of the Australian sea urchin Holopneustes purpurascens. Mar Ecol Prog Ser 314:1–14
Tamburri MN, Zimmer-Faust RK, Tamplin ML (1992) Natural sources and properties of chemical inducers mediating settlement of oyster larvae: a re-examination. Biol Bull 183:327–338
Tamburri MN, Finelli CM, Whethey DS, Zimmer-Faust RK (1996) Chemical induction of larval settlement behavior in flow. Biol Bull 191:367–373
Thompson TE (1958) The natural history, embryology, larval biology, and post-larval development of Adalaria proxima (Alder & Hancock) (Gastropoda Opisthobranchia). Phil Trans Roy Soc London B 242:1–58
Toonen RJ, Pawlik JR (1994) Foundations of gregariousness. Nature 370:511–512
Toonen RJ, Pawlik JR (1996) Settlement of the tube worm Hydroides dianthus (Polychaeta: Serpulidae): cues for gregarious settlement. Mar Biol 126:725–733
Toonen RJ, Pawlik JR (2001) Settlement of the gregarious tube worm Hydroides dianthus (Polychaeta: Serpulidae). I. Gregarious and nongregarious settlement. Mar Ecol Prog Ser 224:103–114
Turner EJ, Zimmerfaust RK, Palmer MA, Lucenbach M, Pentcheff ND (1994) Settlement of oyster (Crassostrea virginica) larvae: effect of water flow and a water soluble chemical cue. Limnol Oceanogr 39:179–1593
Unabia CRC, Hadfield MG (1999) Role of bacteria in larval settlement and metamorphosis of the polychaete Hydroides elegans. Mar Biol 133:55–64
Walters LJ, Hadfield MG, del Carmen KA (1997) The importance of larval choice and hydrodynamics in creating aggregations of Hydroides elegans (Polychaeta: Serpulidae). Invertebr Biol 116:102–114
Watanabe N, Watanabe S, Ide J, Watanabe Y, Sakata K, Okamoto K (1998) Chemical signals involved in larval metamorphosis in Hydroides ezoensis (Serpulidae; Polychaeta). Part II: isolation and identification of a new monoacyl glycerol from adult tube clumps as a metamorphosis-inducing substance. J Mar Biotechnol 6:11–15
Wilson DP (1952) The influence of the nature of the substratum on the metamorphosis of the larvae of marine animals, especially the larve of Ophelia bicornis Savigny. Ann Inst Oceanogr 27:49–156
Wilson DP (1953a) The settlement of Ophelia bicornis Savigny larvae. The 1951 experiments. J Mar Biol Assoc UK 31:413–429
Wilson DP (1953b) The settlement of Ophelia bicornis Savigny larvae. The 1952 experiments. J Mar Biol Assoc UK 32:209–233
Wilson DP (1954) The attractive factor in the settlement of Ophelia bicornis Savigny. J Mar Biol Assoc UK 33:361–380
Wilson DP (1955) The role of micro-organisms in the settlement of Ophelia bicornis Savigny. J Mar Biol Assoc UK 34:531–543
Wisely B (1958) The development of a serpulid worm, Hydroides norvegica, Gunnerus (Polychaeta). Aus J Mar Fresh Res 9:351–361
Zhao B, Qian P-Y (2002) Larval settlement and metamorphosis in the slipper limpet Crepidula onyx (Sowerby) in response to conspecific cues and the cues from biofilm. J Exp Mar Biol Ecol 269:39–51
Zimmer-Faust RK, Tamburri MN, Decho AW (1996) Chemosensory ecology of oyster larvae: benthic-pelagic coupling. In: Lenz PH, Hartline DK, Purcell JE, MacMillan DL (eds) Zooplankton: sensory ecology and physiology. Gordon and Breach, Amersterdam, pp 37–50
Acknowledgements
This research was supported by funds from NSF Grant IOS-0842681 and ONR Grants N00014-05-1-0579 and N00014-08-1-0413 to M. G. H., and from NSF Grant IOS-0842685 to M. A. R. K. We thank T. Cooper for technical assistance. M. G. H. and M. A. R. K. are extremely grateful to the Helen R. Whiteley Center at the Friday Harbor Laboratories of the University of Washington for providing an environment more conducive to evaluating data and writing research papers than any others we know.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Grassle.
Rights and permissions
About this article
Cite this article
Hadfield, M.G., Nedved, B.T., Wilbur, S. et al. Biofilm cue for larval settlement in Hydroides elegans (Polychaeta): is contact necessary?. Mar Biol 161, 2577–2587 (2014). https://doi.org/10.1007/s00227-014-2529-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2529-0