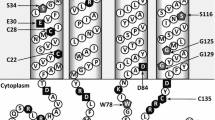
Abstract.
The melibiose carrier of Escherichia coli is a cytoplasmic membrane protein that mediates the cotransport of galactosides with H+, Na+, or Li+. In this study we used cysteine-scanning mutagenesis to try to gain information about the position of transmembrane helix VI in the three-dimensional structure of the melibiose carrier. We constructed 23 individual cysteine substitutions in helix VI and an adjacent loop of the carrier. The resulting melibiose carriers retained 22–100% of their ability to transport melibiose. We tested the effect of the hydrophilic sulfhydryl reagent p-chloromercuri-benzenesulfonic acid (PCMBS) on the cysteine-substitution mutants and we found that there was no inhibition of melibiose transport in any of the mutants. We suggest that helix VI is imbedded in phospholipid and does not face the aqueous channel through which melibiose passes.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 6 March 2001/Revised: 14 May 2001
Rights and permissions
About this article
Cite this article
Ding, P., Weissborn, A. & Wilson, T. Cysteine Substitutions for Individual Residues in Helix VI of the Melibiose Carrier of Escherichia coli . J. Membrane Biol. 183, 33–38 (2001). https://doi.org/10.1007/s00232-001-0053-x
Issue Date:
DOI: https://doi.org/10.1007/s00232-001-0053-x