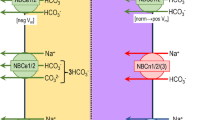
Abstract
The mechanism of epithelial fluid transport is controversial and remains unsolved. Experimental difficulties pose obstacles for work on a complex phenomenon in delicate tissues. However, the corneal endothelium is a relatively simple system to which powerful experimental tools can be applied. In recent years our laboratory has developed experimental evidence and theoretical insights that illuminate the mechanism of fluid transport across this leaky epithelium. Our evidence points to fluid being transported via the paracellular route by a mechanism requiring junctional integrity, which we attribute to electro-osmotic coupling at the junctions. Fluid movements can be produced by electrical currents. The direction of the movement can be reversed by current reversal or by changing junctional electrical charges by polylysine. Aquaporin 1 (AQP1) is the only AQP present in these cells, and its deletion in AQP1 null mice significantly affects cell osmotic permeability but not fluid transport, which militates against the presence of sizable water movements across the cell. By contrast, AQP1 null mice cells have reduced regulatory volume decrease (only 60% of control), which suggests a possible involvement of AQP1 in either the function or the expression of volume-sensitive membrane channels/transporters. A mathematical model of corneal endothelium predicts experimental results only when based on paracellular electro-osmosis, and not when transcellular local osmosis is assumed instead.
Our experimental findings in corneal endothelium have allowed us to develop a novel paradigm for this preparation that includes: (1) paracellular fluid flow; (2) a crucial role for the junctions; (3) hypotonicity of the primary secretion; (4) an AQP role in regulation and not as a significant water pathway. These elements are remarkably similar to those proposed by the Hill laboratory for leaky epithelia.














Similar content being viewed by others
References
Bai C., Fukuda N., Song Y., Ma T., Matthay M.A., Verkman A.S. 1999. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J. Clin. Invest. 103:555–561
Benga G., Popescu O., Borza V., Pop V.I., Muresan A., Mocsy I., Brain A., Wrigglesworth J.M. 1986a. Water permeability in human erythrocytes: identification of membrane proteins involved in water transport. Eur. J. Cell Biol. 41:252–262
Benga G., Popescu O., Pop V.I., Holmes R.P. 1986b. p-(Chloromercuri) benzenesulfonate binding by membrane proteins and the inhibition of water transport in human erythrocytes. Biochemistry (Mosc). 25:1535–1538
Bonanno J.A. 1994. Bicarbonate transport under nominally bicarbonate-free conditions in bovine corneal endothelium. Exp. Eye Res. 58:415–421
Cowan C.A., Yokoyama N., Bianchi L.M., Henkemeyer M., Fritzsch B. 2000. EphB2 guides axons at the midline and is necessary for normal vestibular function. Neuron 26:417–430
Denker B.M., Smith B.L., Kuhajda F.P., Agre P. 1988. Identification, purification and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 263:15634–15642
Diamond J.M., Bossert W.H. 1967. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J. Gen. Physiol. 50:2061–2083
Diecke F.P., Wen Q., Kong J., Kuang K., Fischbarg J. 2004. Immunocytochemical localization of Na+-HCO3- cotransporters in fresh and cultured bovine corneal endothelial cells. Am. J. Physiol. 286:C1434–C1442
Dikstein S., Maurice D.M. 1972. The metabolic basis of the fluid pump in the cornea. J. Physiol. 221:29–41
Doughty M.J., Maurice D.M. 1988. Bicarbonate sensitivity of rabbit corneal endothelium fluid pump in vitro. Invest. Ophthalmol. Vis. Sci. 29:216–223
Fischbarg J. 1995. A rapidly emerging field: water channel proteins in the eye. Invest. Ophthalmol. Vis. Sci. 36:758–763
Fischbarg J. 1997. Mechanism of fluid transport across corneal endothelium and other epithelial layers: a possible explanation based on cyclic cell volume regulatory changes. Br. J. Ophthalmol. 81:85–89
Fischbarg J. 2003. On the mechanism of fluid transport across corneal endothelium and epithelia in general. J. Exp. Zool. A 300:30–40
Fischbarg J., Diecke F.P. 2005. A mathematical model of electrolyte and fluid transport across corneal endothelium. J. Membrane Biol. 203:41–56
Fischbarg J., Liebovitch L.S., Koniarek J.P. 1985. A central role for cell osmolarity in isotonic fluid transport across epithelia. Biol. Cell. 55:239–244
Fischbarg J., Lim J.J. 1974. Role of cations, anions and carbonic anhydrase in fluid transport across rabbit corneal endothelium. J. Physiol. 241:647–675
Fischbarg J., Lim J.J., Bourguet J. 1977. Adenosine stimulation of fluid transport across rabbit corneal endothelium. J. Membrane Biol. 35:95–112
Fischbarg, J., Rubashkin, A., Iserovich, P. 2004. Electro-osmotic coupling in leaky tight junctions is theoretically possible. Experimental Biology 2004. pp. A710 (Abstract #463.6). FASEB, Washington, D.C
Fromter E., Diamond J.M. 1972. Route of passive ion permeation in epithelia. Nature New Biology 235:9–13
Hamann S., Zeuthen T., La Cour M., Nagelhus E.A., Ottersen O.P., Agre P., Nielsen S. 1998. Aquaporins in complex tissues: distribution of aquaporins 1–5 in human and rat eye. Am. J. Physiol. 274:C1332–C1345
Hartmann T., Verkman A.S. 1990. Model of ion transport regulation in chloride-secreting airway epithelial cells. Integrated description of electrical, chemical, and fluorescence measurements. Biophys. J. 58:391–401
Hemlin M. 1995. Fluid flow across the jejunal epithelia in vivo elicited by d-c current: effects of mesenteric nerve stimulation. Acta Physiol. Scand. 155:77–85
Hill A.E. 1975a. Solute-solvent coupling in epithelia: a critical examination of the standing-gradient osmotic flow theory. Proc. R. Soc. Lond. B 190:99–114
Hill A.E. 1975b. Solute-solvent coupling in epithelia: an electro-osmotic theory of fluid transfer. Proc. R. Soc. London B 190:115–134
Hill A.E., Shachar-Hill B., Shachar-Hill Y. 2004. What are aquaporins for? J. Membrane Biol. 197:1–32
Hodson S., Miller F. 1976. The bicarbonate ion pump in the endothelium which regulates the hydration of the rabbit cornea. J. Physiol. 263:563–577
Hogben C.A.M. 1960. Movement of material across cell membranes. The Physiologist 3(4):56–62
Katchalsky A., Curran P.F. 1965. Nonequilibrium Thermodynamics in Biophysics. Harvard University Press, Cambridge, MA
Kovbasnjuk O., Leader J.P., Weinstein A.M., Spring K.R. 1998. Water does not flow across the tight junctions of MDCK cell epithelium. Proc. Natl. Acad. Sci. USA 26:6526–6530
Kuang K., Cragoe E.J., Fischbarg J. 1993. Fluid transport and electroosmosis across corneal endothelium. In: H.H. Ussing, J. Fischbarg, E. Sten Knudsen, E. Hviid Larsen, N.J. Willumsen, editors, Proc. Alfred Benzon Symposium 34, “Water transport in leaky epithelia”. Munksgaard, Copenhagen pp. 69–79
Kuang K., Xu M., Koniarek J.P., Fischbarg J. 1990. Effects of ambient bicarbonate, phosphate and carbonic anhydrase inhibitors on fluid transport across rabbit corneal endothelium. Exp. Eye Res. 50:487–493
Kuang K., Yiming M., Wen Q., Li Y., Ma L., Iserovich P., Verkman A.S., Fischbarg J. 2004. Fluid transport across cultured layers of corneal endothelium from Aquaporin-1 null mice. Exp. Eye Res. 78:791–798
Latta R., Clausen C., Moore L.C. 1984. General method for the derivation and numerical solution of epithelial transport models. J. Membrane Biol. 82:67–82
Lew V.L., Ferreira H.G., Moura T. 1979. The behaviour of transporting epithelial cells. I. Computer analysis of a basic model. Proc. R. Soc. Lond. B. 206:53–83
Liebovitch L.S., Weinbaum S. 1981. A model of epithelial water transport. The corneal endothelium. Biophys. J. 35:315–338
Lim J.J., Liebovitch L.S., Fischbarg J. 1983. Ionic selectivity of the paracellular shunt path across rabbit corneal endothelium. J. Membrane Biol. 73:95–102
Lyslo A., Kvernes S., Garlid K., Ratkje S.K. 1985. Ionic transport across corneal endothelium. Acta Ophthalmol. (Copenh). 63:116–125
Ma T., Fukuda N., Song Y., Matthay M.A., Verkman A.S. 2000. Lung fluid transport in aquaporin-5 knockout mice [see comments]. J. Clin. Invest. 105:93–100
Ma T., Song Y., Gillespie A., Carlson E.J., Epstein C.J., Verkman A.S. 1999. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J. Biol. Chem. 274:20071–20074
McLaughlin S., Mathias R.T. 1985. Electro-osmosis and the reabsorption of fluid in renal proximal tubules. J. Gen. Physiol. 85:699–728
Moore M., Ma T., Yang B., Verkman A.S. 2000. Tear secretion by lacrimal glands in transgenic mice lacking water channels AQP1, AQP3, AQP4 and AQP5. Exp. Eye Res. 70:557–562
Naftalin R.J., Tripathi S. 1985. Passive water flows driven across the isolated rabbit ileum by osmotic, hydrostatic and electrical gradients. J. Physiol. 360:27–50
Narula P.M., Xu M., Kuang K., Akiyama R., Fischbarg J. 1992. Fluid transport across cultured bovine corneal endothelial cell monolayers. Am. J. Physiol. 262:C98–C103
Nielsen R. 1990. Isotonic secretion via frog skin glands in vitro. Water secretion is coupled to the secretion of sodium ions. Acta Physiol. Scand. 139:211–221
Nielsen S., Smith B.L., Christensen E.I., Agre P. 1993a. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA 90:7275–7279
Nielsen S., Smith B.L., Christensen E.I., Knepper M.A., Agre P. 1993b. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J. Cell Biol. 120:371–383
Novotny J.A., Jakobsson E. 1996. Computational studies of ion-water flux coupling in the airway epithelium. I. Construction of model. Am. J. Physiol. 270:C1751–C1763
Ogilvie J.T., McIntosh J.R., Curran P.F. 1963. Volume flow in a series-membrane system. Biochim. Biophys. Acta 66:441–444
Oshio K., Watanabe H., Song Y., Verkman A.S., Manley G.T. 2005. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 19:76–78
Patil R.V., Han Z., Yiming M., Yang J., Iserovich P., Wax M.B., Fischbarg J. 2001. Fluid transport by human nonpigmented ciliary epithelial layers in culture: a homeostatic role for aquaporin-1. Am. J. Physiol. 281:C1139–C1145
Preston G.M., Agre P. 1991. Isolation of the cDNA for erythrocyte membrane protein of 28 kilodaltons: member of an ancient channel familiy. Proc. Natl. Acad. Sci. USA 88:11110–11114
Preston G.M., Carroll W.B., Guggino W.B., Agre P. 1992. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256:385–387
Preston G.M., Smith B.L., Zeidel M.L., Moulds J.J., Agre P. 1994. Mutations in aquaporin-1 in phenotypically normal humans without functional CHIP water channels. Science 265:1585–1587
Reuss L. 2000. General principles of water transport. In: D.W. Seldin, G. Giebisch, editors, The Kidney, Physiology and Pathophysiology (chapter 13). Raven Press, New York pp. 321–340
Reuss, L. 2006. Mechanisms of water transport across cell embranes and epithelia. In: The Kidney, Physiology and Pathophysiology. R.J. Alpern and S.C. Hebert, editors. Elsevier, Amsterdam
Rubashkin, A., Iserovich, P., Hernandez, J., Fischbarg, J. 2005. Epithelial fluid transport: protruding macromolecules and space charges can bring about electro-osmotic coupling at the tight junctions. J. Membrane Biol. 208:251–263.
Sanchez J.M., Li Y., Rubashkin A., Iserovich P., Wen Q., Ruberti J.W., Smith R.W., Rittenband D., Kuang K., Diecke F.P.J., Fischbarg J. 2002. Evidence for a Central Role for Electro-Osmosis in Fluid Transport by Corneal Endothelium. J. Membrane Biol. 187:37–50
Schnermann J., Chou C.L., Ma T., Traynor T., Knepper M.A., Verkman A.S. 1998. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc. Natl. Acad. Sci. USA 95:9660–9664
Shachar-Hill B., Hill A.E. 2002. Paracellular fluid transport by epithelia. Int. Rev. Cytol. 215:319–350
Song Y., Sonawane N., Verkman A.S. 2002. Localization of aquaporin-5 in sweat glands and functional analysis using knockout mice. J. Physiol. 541:561–568
Spring K.R. 1998. Routes and mechanism of fluid transport by epithelia. Annu. Rev. Physiol. 60:105–119
Spring K.R., Paganelli C.V. 1972. Sodium flux in Necturus proximal tubule under voltage clamp. J. Gen. Physiol. 60:181–201
Stamer W.D., Peppel K., O’Donnell M.E., Roberts B.C., Wu F., Epstein D.L. 2001. Expression of aquaporin-1 in human trabecular meshwork cells: role in resting cell volume. Invest. Ophthalmol. Vis. Sci. 42:1803–1811
Timbs M.M., Spring K.R. 1996. Hydraulic properties of MDCK cell epithelium. J. Membrane Biol. 153:1–11
Tripathi S., Boulpaep E.L. 1988. Cell membrane water permeabilities and streaming currents in Ambystoma proximal tubule. Am. J. Physiol. 255:F188–F203
Ussing H.H., Eskesen K. 1989. Mechanism of isotonic water transport in glands. Acta Physiol. Scand. 136:443–454
Van Itallie C.M., Anderson J.M. 2004. The molecular physiology of tight junction pores. Physiology 19:331–338
Van Os C.H., Michels J.A., Slegers J.F. 1976. Effects of electrical gradients on volume flow across gall bladder epithelium. Biochim. Biophys. Acta 443:545–555
Verkman A.S. 2000. Physiological importance of aquaporins: lessons from knockout mice. Curr. Opin. Nephrol. Hypertens. 9:517–522
Verkman A.S., Alpern R.J. 1987. Kinetic transport model for cellular regulation of pH and solute concentration in the renal proximal tubule. Biophys. J. 51:533–546
Verkman A.S., Yang B., Song Y., Manley G.T., Ma T. 2000. Role of water channels in fluid transport studied by phenotype analysis of aquaporin knockout mice. Exp. Physiol. 85 Spec No:233S–241S
Wedner H.J., Diamond J.M. 1969. Contributions of unstirred-layer effects to apparent electrokinetic phenomena in the gallbladder. J. Membrane Biol. 1:92–108
Weinstein A.M., Windhager E.E. 2001. The paracellular shunt of proximal tubule. J. Membrane Biol. 184:241–245
Wen Q., Diecke F.P., Iserovich P., Kuang K., Sparrow J., Fischbarg J. 2001. Immunocytochemical localization of aquaporin-1 in bovine corneal endothelial cells and keratocytes. Exp. Biol. Med. (Maywood) 226:463–467
Whittembury G., Reuss L. 1992. Mechanisms of coupling of solute and solvent transport in epithelia. In: D.W. Seldin, G. Giebisch, editors, The Kidney: Physiology and Pathophysiology. Raven Press, New York pp. 317–360
Acknowledgement
This work was supported by NIH Grant EY06178, and by Research to prevent Blindness, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fischbarg, J., Diecke, F., Iserovich, P. et al. The Role of the Tight Junction in Paracellular Fluid Transport across Corneal Endothelium. Electro-osmosis as a Driving Force. J Membrane Biol 210, 117–130 (2006). https://doi.org/10.1007/s00232-005-0850-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-005-0850-8