Abstract
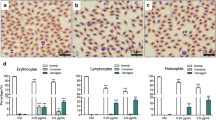
Lead is a multiple-source pollutant, well known for its toxicity, of great risk both for the environment and human health. The main target organs of lead are the hematopoietic, nervous, and renal systems; there are also reports in support of its impairment effects on the reproductive and immune systems. It is well known that most of the metal is accumulated in the blood cells and that many of the deleterious effects are related to its circulating concentrations. These adverse effects have been described not only in humans but also in a number of other vertebrates such as fish and birds. The purpose of the present work was to evaluate the effects of weekly administration of sublethal Pb (as acetate, 50 mg ċ kg−1) during 6 weeks on the profile of the serum proteins and blood cell counts of the adult South American toad, Bufo arenarum (Anura: Bufonidae). The electrophoretic patterns of serum proteins pointed out the presence of four fractions; the metal provoked a significant decrease in both total proteins and albumin fraction; among the globulin fractions, the G3 resulted augmented. These findings may be related to the impact of lead on the toads’ hepatic cells and immune system. The number of total red blood cells (RBC) showed a tendency to decrease after the injections of the metal, whereas the number of white blood cells (WBC) increased significantly; the differential leukocyte counts showed a statistically significant increase in the absolute number and in the relative percentage of blast-like cells. The decrease in RBC was attributed to the negative impact of the metals on the hemoglobin synthesis. The increasing of the WBC counts may be interpreted as a consequence of the induction of proliferation of pluripotential hematopoietic cells.

Similar content being viewed by others
References
Albert LA, Badillo F (1991) Environmental lead in Mexico, Rev Environ Contain Toxicol 117:1–49
Arrieta MA, Peri SI, Apartín C, Rosenberg CE, Fink NE, Salibián A (2000a) Blood lead concentration and δ-aminolevulinic acid dehydratase activity in adult Bufo arenarum. Arch Physiol Biochem 108:275–280
Arrieta MA, Rosenberg CE, Fink NE, Salibián A (2000b) Toxicidad aguda del plomo para Bufo arenarum a dos temperaturas. Acta Toxicol Argentina 8:35
Arrieta MA, Apartín C, Rosenberg CE, Fink NE, Salibián A (2001) Blood lead content in a peri-urban population of the South American toad Bufo arenarum. Sci Total Environ 271:99–195
Arrieta MA, Bruzzone L, Apartin C, Rosenberg CE, Fink NE, Salibián A (2004) Biosensors of inorganic lead exposure and effect in an adult amphibian. Arch Environ Contain Toxicol 46:224–230
Barrett WC Jr (1947) The effect of lead salts on the hemopoietic and histiocytic systems of the larval frog. Am J Anat 81:117–136
Beeby A, Richmond L (2001) Intraspecific competition in populations of Helix aspersa with different histories of exposureto lead. Environ Pollut 114:337–344
Bergdahl IA, Sheveleva M, Sehutz A, Artamonova VG, Skerfving S (1998) Plasma and blood lead in humans: capacity-limited binding to δ-aminolevulinic acid dehydratase and other lead-binding components. Toxicol Sci 46:247–253
Bertini F, Cei JM (1960) Observaciones electroforéticas en seroproteínas de poblaciones argentinas de Bufo arenaruni. Hensel. Rev Soc Argen Biol 36:355
Berzins DW, Bundy KJ (2002) Bioaccumulation of lead in Xenopus laevis tadpoles from water and sediment, Environ Internal 28:66–77
Birdsall CW, Grue CE, Anderson A (1986) Lead concentration in bullfrog Rana catesbeiana and green frog R. clamitans tadpoles inhabiting highway drainages. Environ Pollut Series A 40:233–247
Boutilier RG, Stiffler DF, Toews DP (1992) Exchange of respiratory gases, ions, and water in amphibious and aquatic amphibians. In: Feder ME, Burggren WW (eds) Environmental physiology of the amphibians University of Chicago Press, Chicago, pp 81–124
Boyer R, Grue CE (1995) The need for water quality criteria for frogs, Environ Health Persp 103:352–357
Calderón-Salinas JV, Valdez-Anaya B, Mazúñiga Ch, Albores-Medina A (1996) Lead exposure in a population of Mexican children. Hum Exper Toxicol 15:305–311
Campana O, Sarasquete C, Blasco J (2003) Effect of lead on ALA-D activity, metallothionein levels, and lipid peroxidation in blood, kidney, and liver of the toadfish Halobatrachus didactyfas. Ecotoxicol Environ Saf 55:116–125
Cárdenas A, Roels H, Bernard AM, Barbon R, Buchet JP, Lauwerys RR, et al. (1993) Markers of early renal changes induced by industrial pollutants: II, Application to workers exposed to lead. Br J Ind Med 50:28–36
Carey C, Cohen N, Rollins-Smith L (1999) Amphibian declines: an immunological perspective. Dev Comp Immunol 23:459–472
Carmena-Suero A, Siret JR, Callejas J, Arpones-Carmena D (1980) Blood volume in male Hyla septentronialis (tree frog) and Rana catesbeiana (bullfrog). Comp Biochem Physiol 67A:187–89
Chalumeau-Le Foulgoc MT, Gallien CL (1967) Recherches comparatives sur les protéines sériques dans le genre Pleurodeles (Amphibien urodele). Comp Biochem Physiol 23:679–689
Chiesa M, Rosenberg CE, Arrieta M, Fink N, Salibián A (1999) Efecto de la intoxicacjon crónica por plomo sobre el perfil de las proteínas séricas de anfibio. Medicina (Buenos Aires) 59:624
Christin MS, Gendron AD, Brousseau P, Ménard L, Marcogliese DJ, Cyr D, Ruby S, Fournier M (2003) Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasite infection. Environ Toxicol Chem 22:1127–1133
Clesceri LS, Greenberg AE, Eaton AM (eds) (1998) APHA-AWWA-WPCF; Standard methods for the examination of water and wastewater, Maryland
Conner EA, Fowler BA (1994) Preliminary purification and partial characterization studies of a low-molecular weight cytosolic lead-binding protein in liver of the channel catfish (Ictahirus punctatus).Aquat Toxicol 28:29–36
Daniell WE, Stockbridge HL, Labbe RF, Woods JS, Anderson KE, Bissell DM, et al. (1997) Environmental chemical exposures and disturbances of heme synthesis. Environ Health Persp 105(suppl):37–53
Davic RD, Gallati WW (1979) Erythrocyte number in three species of northern appalachian Desmognathus (Amphibia, Urodela, Plethodontidae). J Herpetol 13:354–356
Dawson AB (1933) An experimental study of hemopoiesis in Necturus: effects of lead poisoning on normal and splenectomized animals. J Morphol 55:349–375
Devilliers J, Exbrayat JM. (eds) (1992) Ecotoxicity of chemicals to amphibians; handbook of ecotoxicological data. Gordon and Breach Science Publishers, Reading, Great Britain, vol. I
Duelman WE, Trueb L (1994) Biology of amphibians. The Johns Hopkins University Press, Baltimore
Eisinger J (1978) Biochemistry and measurement of environmental lead intoxication. Q Revs Biophys 11:439–466
Fink NE, Salibián A (2005) Toxicological studies in adult amphibians: effects of lead. Appl Herpetol 2:311–334
Flores J, Albert LA (2004) Environmental lead in Mexico, 1990–2002. Rev Environ Contam Toxicol 181:37–109
Fowler BA, DuVal G (1991) Effects of lead on the kidney: roles of high-affinity lead-binding proteins. Environ Health Perspect 91:77–80
Fowler BA, Squibb KS (1997) Other metals. In: Goldstein RS (ed), Comprehensive toxicology, volume 7, Elsevier
García Fernández JC, Villaamil EC, Caporaletti G, et al. (1990) Contaminazioni ambientale da piombo: studio comparativo tra gli anni 1975 e 1986 in una zona della “Grande Buenos Aires.” Amb Ris Salute 10:30–33
Goering PL, Fisher BR (1995) Metals and stress proteins. In: Goyer RA, Cherian MG (eds) Toxicology of metals. Biochemical aspects. Springer Verlag, Berlin
Hadji-Azimi I, Coosemans V, Canicatti C (1987) Atlas of adult Xenopus laevis laevis hematology. Dev Comp Immunol 11:807–874
Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL (2000) Quantitative evidence for global amphibian population declines, Nature 404:752–755
IARC (1987) Overall evaluations of carcinogenicity: an updating of IARC monographs 1 to 42. Monogr Eval Carcinog Risks Hum (Suppl):230–231
Jemal A, Graubard BI, Devesa SS, Flegal KM (2002) The association of blood lead level and cancer mortality among whites in the United States. Environ Health Persp 110:325–329
Kaplan HM, Arnholt TJ, Payne JE (1967) Toxicity of lead nitrate solutions for frogs (Rana pipiens) Lab Animal Care 17:240–246
Linder G, Grillitsch B (2000) Ecotoxicology of metals. In: Sparling DW, Linder G, Bishop CA (eds) Ecotoxicology of amphibians and reptiles. Society of Environmental Toxicology and Chemistry (SETAC) Press, Pensacola, Florida
Lips K, Young B, Ibañez R, Salas A (2000) Amphibian declines in Latin America, Froglog 37:1–4
Lloyber I, Schinitman NI, Londero HF (1967) Efectos de la anestesia etérea y de la destruction medular sobre constantes hemocirculatorias del Bufo arenarum Hensel. Rev Soc Arg Biol 43:278–285
López CM, Piñeiro AE, Núñez N, Avagnina AM, Villaamil EC, Roses OE (2000) Thyroid hormone changes in males exposed to lead in the Buenos Aires area (Argentina). Pharmacol Res 42:599–602
Mañay N, Pereira L, Cousillas Z (1999) Lead contamination in Uruguay, Rev Environ Contain Toxicol 159:25–39
Mann R, Bidwell J (1999) Toxicological issues for amphibians in Australia, In: Campbell A (ed) Declines and disappearances of Australian frogs. Canberra, Environment Australia, p 185–201
Mateo R, Hoffman DJ (2001) Differences in oxidative stress between young Canada geese and mallards exposed to lead-contaminated sediment. J Toxicol Environ Health 64A:531–545
McMurry ST, Lochmiller RL, Chandra SAM, Quails CW (1995) Sensitivity of selected immunological, hematological, and reproductive parameters in the cotton rat (Sigmodon hispidus) to subchronic lead exposure. J Wild Dis 31:193–204
Mishra KP, Singh VK, Rani R, Yadav VS, Chandran V, Srivastava SP, Seth PK (2003) Effect of lead exposure on the immune response of some occupationally exposed individuals. Toxicology 188:251–259
Newman MC, Unger MA (2003) Fundamentals of ecotoxicology. Lewis Publisher, Boca Raton, Florida
Paoliello MMB, De Capitani EM (2005) Environmental contamination and human exposure to Lead in Brazil Rev. Environ Contain Toxicol 184:59–96
Perí SI, Arrieta MA, Fink NE, Salibián A (1998a) Delta-atninolevulinic acid dehydratase (ALAD) activity in blood of Bufo arenarum (Amphibia, Anura). Biol Res 31:339–342
Perí SI, Fink NE, Salibián A (1998b) Hematological parameters in Bufo arenarum injected with sublethal dose of Pb acetate. Biomed Environ Sci 11:70–74
Petrakis PL, Brown CW (1970) A high order of heterogeneity in the serum albumin of Ensatina eschscholtzi, a Pacific coast salamander. Comp Biochem Physiol 32:475–487
Quintanilla-Vega B, Smith DR, Kahng MW, Hernández JM, Albores A, Fowler BA (1995) Lead-binding proteins in brain tissue of environmentally lead-exposed humans. Chem Biol Interact 98:193–209
Razani-Boroujerdi S, Edwards B, Sopori ML (1999) Lead stimulates lymphocyte proliferation through enhanced T cell-B cell interaction. J Pharmacol Therap 288:714–719
Rollins-Smith LA (1998) Metamorphosis and the amphibian immune system, Immunol Revs 166:221–230
Romieu Y, Lacasana M, MoConnell R, Pb Research Group of the PAHO (1997) Pb exposure in Latin America and the Caribbean, Environ Health Persp 105:398–405
Ron SR, Merino A (2000) Amphibian declines in Ecuador: overview and first report of chytridiomycosis from South America, Froglog 42:2–3
Rosenberg CE (2001) Estudios hematológicos e inmunológicos en Bufo arenarum (Amphibia; Anura) expuesto al plomo, Doctoral Dissertation, Universidad National de La Plata. La Plata, Argentina
Rosenberg CE, Perí SI, Arrieta MA, Fink NE, Salibián A (1998) Red blood cell osmotic fragility in Bufo arenarum exposed to Pb. Arch Physiol Biochem 106:1–6
Rosenberg CE, Arrieta MA, Fink NE, Salibián A (2000) Actividad fagocítica-lítica de polimorfonucleares de Bufo arenarum inyectado con plomo, Me’dicina (Buenos Aires) 60:848
Rosenberg CE, Salibián A, Fink NE (2002) An enzyme linked immunosorbent assay for measuring anti-ship red blood cells antibodies in lead-exposed toads, J Pharmacol Toxicol Meth 47:121–122
Rosenberg CE, Fink NE, Arrieta MA, Salibián A (2003) Effect of lead acetate on the in vitro engulfment and killing capability of the toad (Bufo arenamm) neutrophils, Comp Biochem Physiol 136C:225–233
Rouf MA (1969) Henmtology of the leopard frog Rana pipiem, Copeia 4:682–687
Rowe CL, Hopkins WA, Coffman VR (2001) Failed recruitment of Southern toads (Bufo terrestris) in a trace element-contaminated breeding habitat: direct and indirect effects that may lead to a local population sink. Arch Environ Contain Toxicol 40:399–405
Rowe CL, Hopkins WA, Bridges CM (2003) Physiological ecology of amphibians in relation to susceptibility to natural and anthropogenic factors. In: Hinder .G, Crest GS, Sparling DW (eds) Amphibian decline: an integrated analysis of multiple stressor effects. Society of Environmental Toxicology and Chemistry (SETAC) Pensacola, Florida, pp 9–57
Sarkar S (1996) Ecological theory and anuran declines. BioScience 46:199–207
Schuytema GS, Nebeker AV (1996) Amphibian toxicity data for water quality criteria. US EPA, National Health and Environmental Effects Research Laboratory, Western Ecology Division, Corvallis, Oregon (EPA/600/R-96/124)
Silbergeld EK (1995) The hazard of synthetic (anthropogenic) chemicals. Toxicol Lett 82/83:835–841
Stansley W, Roscoe DE (1996) The uptake and effects of lead in small mammals and frogs at a trap and skeet range. Arch Environ Contain Toxicol 30:220–226
Szubarkowska E, Gromysz-Kalkowska K, Wójcik K (1990) Behavior of the formed blood elements in Rana esculenta L. after repeated contacts of the animal with a therapeutic dose of foschlor. Bull Environ Contain Toxicol 45:796–803
Tong S, von Schirnding YE, Prapamontol T (2000) Environmental lead exposure: a public health problem of global dimensions. Bull WHO 78:1068–1077
Valle BL, Ulmer DD (1972) Biochemical effects of mercury, cadmium and lead. Ann Rev Biochem 41:91–128
Varela ME, Sellares ME (1937) Sobre la morfología hemática de Bufo arenarum (Hensel). Rev Soc Argen Biol 13:345–349
Varela ME, Sellarés ME (1938) Nuevas observaciones sobre las variaciones estacionales del cuadro hemático del Bufo arenarum Hensel. Rev Soc Argen Biol 14:229–235
Vogiatzis AK, Loumbourdis NS (1999) Exposure of Rana ridibunda to lead, I. Study of lead accumulation in various tissues and hepatic δ-aminolevulinic acid dehydratase activity. J Appl Toxicol 19:25–29
WHO (1977) Lead. Environmental health Criteria 3. World Health Organization, Geneva 202 pp
WHO (1989) Lead: environmental aspects. Environmental health criteria 85. Geneva, World Health Organization (http://www.inchem.org/documents/ehc/ehc/ehc85.htm; accessed Nov 9, 2004)
WHO (1995) Inorganic lead. Environmental health criteria 165. Geneva, World Health Organization (http://www.inchem.org/documents/ehc/ehc/ehc165.htm; accessed Nov 9, 2004)
Williams GM, Iatropoulos MJ (2002) Alteration of liver cell function and proliferation; differentiation between adaptation and toxicity. Toxicol Pathol 30:41–53
Woods JS (1995) Porphyrin metabolism as indicator of metal exposure and toxicity. In: Goyer RA, Cherian MG (eds) Toxicology of metals. Biochemical aspects. Springer Verlag, Berlin, pp 19–52
Acknowledgments
C.E. Rosenberg was a research Fellow of CIC-Buenos Aires. The authors wish to thank Carina Apartin for her collaboration in the determination of Pb, Marisa Sanguinetti for her assistance in reviewing English language, and Ana M. Martínez for the preparation of the manuscript. This work was supported by the UNLP and the CIC-Buenos Aires. Wiener Lab, Argentina, kindly provided the reagents for serum protein determination.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiesa, M.E., Rosenberg, C.E., Fink, N.E. et al. Serum Protein Profile and Blood Cell Counts in Adult Toads Bufo Arenarum (Amphibia: Anura: Bufonidae): Effects of Sublethal Lead Acetate. Arch Environ Contam Toxicol 50, 384–391 (2006). https://doi.org/10.1007/s00244-004-0252-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-004-0252-4