Abstract
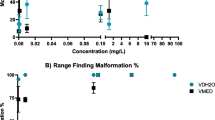
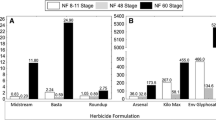
Globally, amphibians are experiencing widespread abnormalities and population declines. One potential contributor to these challenges is the use of pesticides, particularly aquatic herbicides applied to aquatic habitats inhabited by amphibians. Critical issues of concern are the potential toxicity and teratogenicity of these herbicides towards amphibians. Using the FETAX protocol, three globally used formulations, including diquat dibromide (Midstream), glufosinate ammonium (Basta), and imazapyr (Arsenal), were assessed for embryotoxicity, teratogenicity, and growth inhibition. Developing Xenopus laevis embryos were exposed for 96 h at concentrations of 0.5–3.0 mg/L, 1.6–3.0 mg/L, and 20–45 mg/L for Midstream, Basta, and Arsenal respectively. The 96-h LC50 estimates were 0.83 mg/L acid equivalent (a.e.), 36 mg/L a.e., and 2.2 mg/L a.e., whereas the EC50 estimates were 0.24 mg/L a.e., 28.13 mg/L a.e., and 2.01 mg/L a.e. for the Midstream, Arsenal, and Basta formulations, respectively. These two estimates produced Teratogenic Index of 3.5, 1.3, and 1.1 for Midstream, Arsenal, and Basta, respectively, indicating a high risk of malformation induction by Midstream and moderate risk for Arsenal. Regarding growth inhibition, lowest observable effect concentrations of 0.5 mg/L, 25 mg/L, and 2.0 mg/L were computed for Midstream, Arsenal, and Basta, respectively, producing the minimum concentration inhibiting growth (MCIG) ratios of 0.62, 0.69, and 0.89 for the three formulations. These MICG values are higher than the standard 0.30 growth inhibitors benchmark, suggesting that the formulations are not growth inhibitors at the evaluated concentrations. This study provides evidence of the embryotoxic and teratogenic status of Midstream and the embryotoxicity of Basta. There is a need to further characterise the physiological and ecological impacts of these formulations to ensure responsible use and the safety of amphibians and other wildlife.


Similar content being viewed by others
References
American Society for Testing and Materials (2014) Standard guide for conducting frog embryo teratogenesis assay-Xenopus, ASTM E1439-98: annual book of ASTM standards, vol 11.05. ASTM, Philadelphia, PA, pp 826–36
American Society for Testing and Materials (ASTM) (1998) Standard guide for conducting the frog embryo teratogenesis assay-Xenopus (FETAX). E1439-98
Anderson RJ, Prahlad KV (1976) The deleterious effects of fungicides and herbicides on Xenopus laevis embryos. Arch Environ Contam Toxicol 4(3):312–323
Anisuzzaman KM, Amin M, Ogg N, Hoq F, Kanithi MR, Jenkins RE (2000) Synthesis of dimethyl derivatives of imidazolinone herbicides: their use in efficient gas chromatographic methods for the determination of these herbicides. J Agric Food Chem 48(12):5893–5902. https://doi.org/10.1021/jf000428h
Ansara-Ross TM, Wepener V, van den Brink PJ, Ross MJ (2012) Pesticides in South African fresh waters. African J Aquatic Sci 37(1):1–16
Babalola OO, van Wyk JH (2017) Comparative early life stage toxicity of African clawed frog, X. laevis following exposure to selected herbicide formulations applied to eradicate alien plants in South Africa. Arch Environ Contam Toxicol. https://doi.org/10.1007/S00244-017-0463-0
Babalola OO, van Wyk JH (2019) Mortality, teratogenicity and growth inhibition of three glyphosate formulations using frog embryo teratogenesis assay-Xenopus. J Appl Toxicol 2019:1–10. https://doi.org/10.1002/jat.3811
Bantle J, Dumont J, Finch R, Linder G (1999) Atlas of abnormalities: a guide for the performance of FETAX. Oklahoma State Publications Department, Stillwater, OK
Bernardini G, Vismara C, Boracchi P, Camatini M (1994) Lethality, teratogenicity and growth inhibition of heptanol in Xenopus assayed by a modified frog embryo teratogenesis assay: Xenopus (FETAX) procedure. Sci Environ 151:1–8
Bimber DL, Mitchell RA (1978) Effects of diquat on amphibian embryo development. Ohio J Sci 78(1):50–51
Blaustein AR, Wake DB, Sousa WP (1994) Amphibian declines: judging stability, persistence, and susceptibility of populations to local and global extinctions. Conserv Biol 8:60–71
Boga A, Binokay S, Sertdemir Y (2009) The toxicity and teratogenicity of gibberellic acid (GA3) based on the frog embryo teratogenesis assay-Xenopus (FETAX). Turk J Biol 33:181–188
Bold T (2007) Management treatments summary guide: aquatics. Working for Waters National Office. www.dwaf.gov.za/wfw/control. Accessed 16 Jan 2017
Brühl CA, Schmidt T, Pieper S, Alscher A (2013) Terrestrial pesticide exposure of amphibians: An underestimated cause of global decline? Sci Rep 3:1135
Budde WL (2003) Analytical mass spectrometry of herbicides. Mass Spectrom Rev 23(1):1–24. https://doi.org/10.1002/mas.10070
Dawson DA, Bantle JA (1987) Development of a reconstituted water medium and initial validation of FETAX. J Appl Toxicol 7:237–244
Dawson DA, Fort DJ, Smith GJ, Newell DL, Bantle JA (1988) Evaluation of developmental toxicity of nicotine and cotinine with FETAX. Teratogen Carcinogen Mutagen 8:329–338
Dawson DA, Mcmcormick CA, Bantle JA (1985) Detection of teratogenic substances in acidic mine water samples using the Frog Embryo Teratogenesis Assay-Xenopus (FETAX). J Appl Toxicol 5:234–244
de Almeida RM, Yoramine M (2007) Gas chromatography-mass spectrometric method for the determination of the herbicide paraquat and diquat in plasma and urine samples. J Chromatogr B 853:260–264
Dial NA, Dial CAB (1987) Lethal effects of diquat and paraquat on developing frog embryos and 15-day-old R. pipien. Bull Environ Contam Toxicol 38:1006–1011
Downing JA, Cole JJ, Middelburg JJ, Striegl RG, Duarte CM, Kortelainen P, Prairie YT, Laube KA (2008) Sediment organic carbon burial in agriculturally eutrophic impoundments over the last century. Global Biogeochem Cycles 22:GB1018. https://doi.org/10.1029/2006gb002854
Ebert E, Leist KH, Mayer D (1990) Summary of safety evaluation toxicity studies of glufosinate ammonium. Food Chem Toxicol 28:339–349
Eldredge N (1998) Life in the balance: humanity & the biodiversity crisis. Princeton Univ. Press, Princeton, NJ
Emmett K (2002) Final risk assessment for diquat bromide. The Water Quality Program of the Washington State Department of Ecology. 02-10-046
European Environment Agency (EEA) (2011) Safe water and health water services in a changing environment. EEA technical report no. 7. ISSN 1725-2237
Fort DJ, Mathis M (2018) Frog embryo teratogenesis assay—xenopus (FETAX): use in alternative preclinical safety assessment. Cold Spring Harb Protoc. https://doi.org/10.1101/pdb.prot098319
Fort DJ, Paul RR (2002) Enhancing the Predictive Validity of Frog Embryo Teratogenesis Assay—Xenopus (FETAX). J Appl Toxicol 22:185–191. https://doi.org/10.1002/jat.848
Fort DJ, Stower EL, Norton D (1995) Ecological hazard assessment of aqueous soil extracts using FETAX. J Appl Toxicol 15:183–191
Grisolia CK, Bilich MR, Formigli LM (2004) A comparative toxicologic and genotoxic study of the herbicide arsenal, its active ingredient imazapyr, and the surfactant nonylphenol ethoxylate. Ecotoxicol Environ Saf 59:123–126
Gungordu A (2013) Comparative toxicity of methidathion and glyphosate on early life stages of three amphibian species: Pelophylax ridibundus, Pseudepidalea viridis, and Xenopus laevis. Aquat Toxicol 140–141:220–228. https://doi.org/10.1016/j.aquatox.2013.06.012
Koyama K, Koyama K, Goto K (1997) Cardiovascular effects of herbicide containing glufosinate and a surfactant: in vitro and in vivo analysis in rat. Toxicol Appl Pharmacol 145:409–414
Lajmanovich RC, Junges CM, Attademo AM, Peltzer PM, Cabagna-Zenklusen MC, Basso A (2013) Individual and mixture toxicity of commercial formulations containing glyphosate, metsulfuron-methyl, bispyribac-sodium, and picloram on Rhinella arenarum tadpoles. Water Air Soil Pollut 224:1404
Leconte I, Mouche I (2013) Frog embryo teratogenesis assay on Xenopus and predictivity compared with in vivo mammalian studies. Methods Mol Biol 947:403–421
Liu W, Pusino A, Gessa C (1992) High-performance liquid chromatographic determination of the herbicide imazapyr residues in water and soil. Sci Total Environ 123(124):39–43
Liu R, Zhou JL, Wilding A (2004) Microwave-assisted extraction followed by gas chromatography–mass spectrometry for the determination of endocrine disrupting chemicals in river sediments. J Chromatogr A 1038(1–2):19–26. https://doi.org/10.1016/j.chroma.2004.03.030
Mann RM, Bidwell JR (2000) Application of the FETAX protocol to assess developmental toxicity of nonylphenol ethoxylate to Xenopus laevis and two Australian frogs. Aquatic Toxicol 51:19–29
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of risks in a complex environment. Environ Pollut 157:2903–2927
Meinhardt HR (2008) Evaluation of predictive models for pesticide behaviour in South African soils. PhD thesis, University of the North-West, South Africa
Mensah PK, Palmer CG, Muller WJ (2013) Derivation of South African water quality guidelines for Roundups using species sensitivity distribution. Ecotoxicol Environ Saf 96:24–31
Morgan MK, Scheuerman PR, Bishop CS, Pyles RA (1996) Teratogenic potential of atrazine & 2, 4-D using FETAX. J Toxicol Environ Health 48:151–168
National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) (2000) Frog Embryo Teratogenesis Assay-Xenopus-background review document. Retrieved December 12, 2018 from www.niceatm.org
Nieuwkoop PD, Faber J (1956) Normal table of X. laevis. North Holland, Amsterdam
Oberholster PA, Botha KA, Babalola OO, Ndlela L, Staebe K, van Wyk JH (2014) The adverse effects of anthropogenic pollution on xenopus laevis with special reference to Acid Mine Drainage (AMD) in a freshwater Wetland. In M. Lonbardi (Ed.), Amphibian, Anatomy, Ecological Significance and Conservation Strategies. Animal Science, Issues & Professions. Novinka Science Publishers Inc, New York
Organisation for Economic Cooperation and Development (OECD) (2007) Validation of the amphibian metamorphosis assay as a screen for thyroid-active chemicals: integrated AMA summary report. Retrieved November 6, 2018 from www.oecd.org/document
Organisation for Economic Co-operation and Development (OECD) (2008) Series on testing and assessment. No. 91. Report of the validation of the amphibian metamorphosis assay (PHASE 3) ENV/JM/MONO(2008)18. Retrieved November 9, 2018 fromwww.oecd.org/document
Osano O, Oladimeji A, Kraak MS, Admiraal W (2002) Teratogenic effects of amitraz, 2, 4-dimethylaniline, and paraquat on developing (Xenopus) embryos. Arch Environ Contam Toxicol 43:42–49
Othman MZ, Ding L, Jiao Y (2009) Effect of anionic and non-ionic surfactants on activated sludge oxygen uptake rate and nitrification. World Academy of Science, Engineering and Technology 58
Peterson HG, Boutin G, Martin PA, Freemark KE, Ruecker NJ, Moody MJ (1994) Aquatic phyto-toxicity of 23 pesticides applied at expected environmental concentrations. Aquatic Toxicol 28:275–292
Qian H, Chen W, Sheng GD, Xu X, Liu W, Fu Z (2008) Effects of glufosinate on antioxidant enzymes, subcellular structure, and gene expression in unicellular green alga Chlorella vulgaris. Aquatic Toxicol 88:301–307
Schuytema GS, Nebeker AV, Griffis WL (1994) Toxicity of Guithion and Guthion 2S to Xenopus laevis embryos. Arch Environ Contam Toxicol 27:250–255
Selypes A, Nagymojtenyi L, Berensi G (1980) Mutagenic and embryotoxic effects of paraquat and diquat. Bull Environ Contam Toxicol 25:513–517
Shen G, Lee HK (2003) Determination of triazines in soil by microwave-assisted extraction followed by solid-phase microextraction and gas chromatography–mass spectrometry. J Chromatogr A 985(1–2):167–174. https://doi.org/10.1016/S0021-9673(02)01222-0
USEPA (1998) US Environmental Protection Agency - Office of Pollution Prevention and Toxics. Chemical hazard data. Availability study. What do we really know about the safety of high production volume chemicals? Washington DC. Retrieved March 15, 2018 from http://www.epa.gov/HPV/pubs/general/hazchem.htm
Wagner N, Wolfram R, Hanka T, Beatrix T, Stefan L (2013) Questions concerning the potential impact of glyphosate-based herbicides on amphibians. Environ Toxicol Chem 32:1688–1700. https://doi.org/10.1002/etc.2268
Washington State Department of Agriculture (WSDA) (2003) Ecological risk assessment of proposed use of imazapyr to control invasive cordgrass in estuarine habitat of Washington State. Project no. 3000901. Retrieved October 4, 2019 from www.ecy.wa.gov
Washington State Department of Agriculture (WSDA) (2009) Human health and ecological effects imazapyr risk assessment, Washington State. Retrieved October 4, 2019 from www.ecy.wa.gov
Watanabe T, Iwase T (1996) Developmental & dysmorphogenic effects of glufosinate ammonium on mouse embryo in culture. Teratogen Carcinogen Mutagen 16:287–299
World Health Organisation (WHO) (2004) Diquat in drinking water. background document for development of who guidelines for drinking-water quality. World Health Organisation, WHO/SDE/WSH/03.04/91
World Health Organisation (WHO) (2013) State of the Science of Endocrine Disrupting Chemicals (2012). United Nations Environmental Programme and World Health Organisation. ISBN 978-92-807-3274-0 (UNEP) and 978 92 4 150503 1 (WHO)
WWF- CHEM TRUST (2010) Protecting future generations by reducing exposure to endocrine disruptors. CHEM Trust and WWF-EPO proposals for the regulation of chemicals with endocrine disrupting properties under REACH (EC 1907/2006) and under the Plant Protection Products Regulation (EC No 1107/2009)
Yu S, Wages MR, Cai Q, Maul JD, Cobb GP (2013) Lethal and sublethal effects of three insecticides on two developmental stages of Xenopus laevis and comparison with other amphibians. Environ Toxicol Chem 32:2056–2064
Acknowledgements
The authors thank Dr. Olatunde Oladapo, formerly of Zoology and Environmental Biology Department, Lagos State University in Nigeria, for all his support. They wish him happy and good health in retirement.
Funding
This study was supported by the Water Research Commission, South Africa, Research grant (Grant Number K5/1952), as well as the Working for Water Department, Ministry of Water Affairs, South Africa, for the supply of all the herbicides used for this study. The authors declare that both the Water Research Commission and Working for Water Department, both in South Africa, did not in any way contribute to the design of the experiment, data analysis, as well as report writing and choice of publication.
Author information
Authors and Affiliations
Contributions
Babalola performed the laboratory work and manuscript writing. Truter performed all of the statistical and software analysis, and van Wyk performed the general supervision and conceptualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in financial, relationship, or otherwise.
Ethical Approval
The authors declare that all experiments used in this study comply with the current laws in South Africa (Animal Ethics Permit No. SU-ACUM 12-00014).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Babalola, O.O., Truter, J.C. & Van Wyk, J.H. Lethal and Teratogenic Impacts of Imazapyr, Diquat Dibromide, and Glufosinate Ammonium Herbicide Formulations Using Frog Embryo Teratogenesis Assay-Xenopus (FETAX). Arch Environ Contam Toxicol 80, 708–716 (2021). https://doi.org/10.1007/s00244-020-00756-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-020-00756-5