Abstract
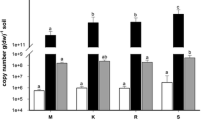
Glacier forefield chronosequences, initially composed of barren substrate after glacier retreat, are ideal locations to study primary microbial colonization and succession in a natural environment. We characterized the structure and composition of bacterial, archaeal and fungal communities in exposed rock substrates along the Damma glacier forefield in central Switzerland. Soil samples were taken along the forefield from sites ranging from fine granite sand devoid of vegetation near the glacier terminus to well-developed soils covered with vegetation. The microbial communities were studied with genetic profiling (T-RFLP) and sequencing of clone libraries. According to the T-RFLP profiles, bacteria showed a high Shannon diversity index (H) (ranging from 2.3 to 3.4) with no trend along the forefield. The major bacterial lineages were Proteobacteria, Actinobacteria, Acidobacteria, Firmicutes and Cyanobacteria. An interesting finding was that Euryarchaeota were predominantly colonizing young soils and Crenarchaeota mainly mature soils. Fungi shifted from an Ascomycota-dominated community in young soils to a more Basidiomycota-dominated community in old soils. Redundancy analysis indicated that base saturation, pH, soil C and N contents and plant coverage, all related to soil age, correlated with the microbial succession along the forefield.





Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Anderson IC, Campbell CD, Prosser JI (2003) Potential bias of fungal 18S rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil. Environ Microbiol 5:36–47
Ayton J, Aislabie J, Barker GM, Saul D, Turner S (2010) Crenarchaeota affiliated with group 1.1b are prevalent in coastal mineral soils of the Ross Sea region of Antarctica. Environ Microbiol 12:689–703
Bernasconi SM, Bauder A, Bourdon B, Brunner I, Bunemann E, Christl I, Derungs N, Edwards P, Farinotti D, Frey B, Frossard E, Furrer G, Gierga M, Goransson H, Gulland K, Hagedorn F, Hajdas I, Hindshaw R, Ivy-Ochs S, Jansa J, Jonas T, Kiczka M, Kretzschmar R, Lemarchand E, Luster J, Magnusson J, Mitchell EAD, Venterink HO, Plotze M, Reynolds B, Smittenberg RH, Stahli M, Tamburini F, Tipper ET, Wacker L, Welc M, Wiederhold JG, Zeyer J, Zimmermann S, Zumsteg A (2011) Chemical and biological gradients along the Damma glacier soil chronosequence, Switzerland. Vadose Zone J 10:867–883
Bernasconi SM, BigLink (2008) Weathering, soil formation and initial ecosystem evolution on a glacier forefield: a case study from the Damma glacier, Switzerland. Mineral Mag 72:19–22
Bintrim SB, Donohue TJ, Handelsman J, Roberts GP, Goodman RM (1997) Molecular phylogeny of archaea from soil. Proc Natl Acad Sci USA 94:277–282
Borneman J, Hartin RJ (2000) PCR primers that amplify fungal rRNA genes from environmental samples. Appl Environ Microbiol 66:4356–4360
Brunner I, Plotze M, Rieder SR, Zumsteg A, Furrer G, Frey B (2011) Pioneering fungi from the Damma glacier forefield in the Swiss Alps can promote granite weathering. Geobiology 9:266–279
Burford EP, Fomina M, Gadd GM (2003) Fungal involvement in bioweathering and biotransformation of rocks and minerals. Mineral Mag 67:1127–1155
Chaban B, Ng SYM, Jarrell KF (2006) Archaeal habitats—from the extreme to the ordinary. Can J Microbiol 52:73–116
Chin KJ, Lukow T, Conrad R (1999) Effect of temperature on structure and function of the methanogenic archaeal community in an anoxic rice field soil. Appl Environ Microbiol 65:2341–2349
Crews TE, Kurina LM, Vitousek PM (2001) Organic matter and nitrogen accumulation and nitrogen fixation during early ecosystem development in Hawaii. Biogeochemistry 52:259–279
Deiglmayr K, Philippot L, Tscherko D, Kandeler E (2006) Microbial succession of nitrate-reducing bacteria in the rhizosphere of Poa alpina across a glacier foreland in the Central Alps. Environ Microbiol 8:1600–1612
Duc L, Noll M, Meier BE, Burgmann H, Zeyer J (2009) High diversity of diazotrophs in the forefield of a receding Alpine glacier. Microb Ecol 57:179–190
Edwards IP, Burgmann H, Miniaci C, Zeyer J (2006) Variation in microbial community composition and culturability in the rhizosphere of Leucanthemopsis alpina (L.) heywood and adjacent bare soil along an alpine chronosequence. Microb Ecol 52:679–692
Fermani P, Mataloni G, Van de Vijver B (2007) Soil microalgal communities on an Antarctic active volcano (Deception Island, South Shetlands). Polar Biol 30:1381–1393
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci USA 103:626–631
Freeman KR, Pescador MY, Reed SC, Costello EK, Robeson MS, Schmidt SK (2009) Soil CO2 flux and photoautotrophic community composition in high-elevation, ‘barren’ soil. Environ Microbiol 11:674–686
Frey B, Kremer J, Rudt A, Sciacca S, Matthies D, Luscher P (2009) Compaction of forest soils with heavy logging machinery affects soil bacterial community structure. Eur J Soil Biol 45:312–320
Frey B, Rieder SR, Brunner I, Ploetze M, Koetzsch S, Lapanje A, Brandl H, Furrer G (2010) Weathering-associated bacteria from the Damma glacier forefield: physiological capabilities and impact on granite dissolution. Appl Environ Microbiol 76:4788–4796
Frey B, Stemmer M, Widmer F, Luster J, Sperisen C (2006) Microbial activity and community structure of a soil after heavy metal contamination in a model forest ecosystem. Soil Biol Biochem 38:1745–1756
Gleeson DB, Clipson N, Melville K, Gadd GM, McDermott FP (2005) Characterization of fungal community structure on a weathered pegmatitic granite. Microb Ecol 50:360–368
Gleeson DB, Kennedy NM, Clipson N, Melville K, Gadd GM, McDermott FP (2006) Characterization of bacterial community structure on a weathered pegmatitic granite. Microb Ecol 51:526–534
Gorbushina AA, Broughton WJ (2009) Microbiology of the atmosphere–rock interface: how biological interactions and physical stresses modulate a sophisticated microbial ecosystem. Annu Rev Microbiol 63:431–450
Hawes TC (2008) Aeolian fallout on recently deglaciated terrain in the high Arctic. Polar Biol 31:295–301
Hestmark G, Skogesal O, Skullerud O (2007) Early recruitment equals long-term relative abundance in an Alpine saxicolous lichen guild. Mycologia 99:207–214
Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH (1997) Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol 63:3233–3241
Hibbett DS, Matheny PB (2009) The relative ages of ectomycorrhizal mushrooms and their plant hosts estimated using Bayesian relaxed molecular clock analyses. BMC Biol 7:13
Hodkinson ID, Coulson SJ, Webb NR (2003) Community assembly along proglacial chronosequences in the high Arctic: vegetation and soil development in north-west Svalbard. J Ecol 91:651–663
Hodkinson ID, Webb NR, Coulson SJ (2002) Primary community assembly on land—the missing stages: why are the heterotrophic organisms always there first? J Ecol 90:569–577
Horath T, Bachofen R (2009) Molecular characterization of an endolithic microbial community in dolomite rock in the Central Alps (Switzerland). Microb Ecol 58:290–306
Huber T, Faulkner G, Hugenholtz P (2004) Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317–2319
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Jia YL, Bhat SG, Fraser MP (2010) Characterization of saccharides and other organic compounds in fine particles and the use of saccharides to track primary biologically derived carbon sources. Atmos Environ 44:724–732
Jumpponen A (2007) Soil fungal communities underneath willow canopies on a primary successional glacier forefront: rDNA sequence results can be affected by primer selection and chimeric data. Microb Ecol 53:233–246
Jumpponen A (2003) Soil fungal community assembly in a primary successional glacier forefront ecosystem as inferred from rDNA sequence analyses. New Phytol 158:569–578
Jumpponen A, Trappe JM (1998) Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol 140:295–310
Kaplan CW, Kitts CL (2003) Variation between observed and true terminal restriction fragment length is dependent on true TRF length and purine content. J Microbiol Methods 54:121–125
Kersters K, De Vos P, Gillis M, Vandamme P, Stackebrandt E (2006) Introduction to the Proteobacteria. Springer, New York
Lazzaro A, Abegg C, Zeyer J (2009) Bacterial community structure of glacier forefields on siliceous and calcareous bedrock. Eur J Soil Sci 60:860–870
Lee MR, Parsons I (1999) Biomechanical and biochemical weathering of lichen-encrusted granite: textural controls on organic-mineral interactions and deposition of silica-rich layers. Chem Geol 161:385–397
Lee SH, Cho JC (2009) Distribution patterns of the members of phylum acidobacteria in global soil samples. J Microbiol Biotechnol 19:1281–1287
Ludley KE, Robinson CH (2008) ‘Decomposer’ basidiomycota in Arctic and Antarctic ecosystems. Soil Biol Biochem 40:11–29
Mannisto MK, Tiirola M, Haggblom MM (2007) Bacterial communities in Arctic fields of Finnish Lapland are stable but highly pH-dependent. FEMS Microbiol Ecol 59:452–465
Miadlikowska J, Kauff F, Hofstetter V, Fraker E, Grube M, Hafellner J, Reeb V, Hodkinson BP, Kukwa M, Lucking R, Hestmark G, Otalora MG, Rauhut A, Budel B, Scheidegger C, Timdal E, Stenroos S, Brodo I, Perlmutter GB, Ertz D, Diederich P, Lendemer JC, May P, Schoch CL, Arnold AE, Gueidan C, Tripp E, Yahr R, Robertson C, Lutzoni F (2006) New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA- and two protein-coding genes. Mycologia 98:1088–1103
Miniaci C, Bunge M, Duc L, Edwards I, Burgmann H, Zeyer J (2007) Effects of pioneering plants on microbial structures and functions in a glacier forefield. Biol Fertil Soils 44:289–297
Muhlmann O, Peintner U (2008) Ectomycorrhiza of Kobresia myosuroides at a primary successional glacier forefront. Mycorrhiza 18:355–362
Nemergut DR, Anderson SP, Cleveland CC, Martin AP, Miller AE, Seimon A, Schmidt SK (2007) Microbial community succession in an unvegetated, recently deglaciated soil. Microb Ecol 53:110–122
Nicol GW, Tscherko D, Chang L, Hammesfahr U, Prosser JI (2006) Crenarchaeal community assembly and microdiversity in developing soils at two sites associated with deglaciation. Environ Microbiol 8:1382–1393
Nicol GW, Tscherko D, Embley TM, Prosser JI (2005) Primary succession of soil crenarchaeota across a receding glacier foreland. Environ Microbiol 7:337–347
Nielsen UN, Osler GHR, Campbell CD, Burslem D, van der Wal R (2010) The influence of vegetation type, soil properties and precipitation on the composition of soil mite and microbial communities at the landscape scale. J Biogeogr 37:1317–1328
Paul F, Kaab A, Maisch M, Kellenberger T, Haeberli W (2004) Rapid disintegration of Alpine glaciers observed with satellite data. Geophys Res Lett 31:L21402
Pearce DA, Bridge PD, Hughes KA, Sattler B, Psenner R, Russell NJ (2009) Microorganisms in the atmosphere over Antarctica. FEMS Microbiol Ecol 69:143–157
Pesaro M, Widmer F, Nicollier G, Zeyer J (2003) Effects of freeze–thaw stress during soil storage on microbial communities and methidathion degradation. Soil Biol Biochem 35:1049–1061
Scheublin TR, Sanders IR, Keel C, van der Meer JR (2010) Characterisation of microbial communities colonising the hyphal surfaces of arbuscular mycorrhizal fungi. ISME J 4:752–763
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Schmidt SK, Reed SC, Nemergut DR, Grandy AS, Cleveland CC, Weintraub MN, Hill AW, Costello EK, Meyer AF, Neff JC, Martin AM (2008) The earliest stages of ecosystem succession in high-elevation (5000 metres above sea level), recently deglaciated soils. Proc R Soc B Biol Sci 275:2793–2802
Schutte UME, Abdo Z, Bent SJ, Williams CJ, Schneider GM, Solheim B, Forney LJ (2009) Bacterial succession in a glacier foreland of the high Arctic. ISME J 3:1258–1268
Schutte UME, Abdo Z, Foster J, Ravel J, Bunge J, Solheim B, Forney LJ (2010) Bacterial diversity in a glacier foreland of the high Arctic. Mol Ecol 19:54–66
Sigler WV, Zeyer J (2004) Colony-forming analysis of bacterial community succession in deglaciated soils indicates pioneer stress-tolerant opportunists. Microb Ecol 48:316–323
Sigler WV, Zeyer J (2002) Microbial diversity and activity along the forefields of two receding glaciers. Microb Ecol 43:397–407
Stibal M, Tranter M, Benning LG, Rehak J (2008) Microbial primary production on an Arctic glacier is insignificant in comparison with allochthonous organic carbon input. Environ Microbiol 10:2172–2178
Ter Braak CJF, Smilauer P (2002) CANOCO reference manual and canodraw for Windows user’s guide: software for canonical community ordination (version 4.5)
Thomas GW (1982) Methods of soil analysis. Part 2. Chemical and microbiological properties. Exchangeable cations, 2nd edn, vol. 9. American Society of Agronomy, Madison
Thomas GW (1996) Methods of soil analysis. Part 3. Chemical methods. Soil pH and soil acidity. Soil Science Society of America, Madison
Timonen S, Bomberg M (2009) Archaea in dry soil environments. Phytochem Rev 8:505–518
Tscherko D, Hammesfahr U, Zeltner G, Kandeler E, Bocker R (2005) Plant succession and rhizosphere microbial communities in a recently deglaciated alpine terrain. Basic Appl Ecol 6:367–383
Widmer F, Hartmann M, Frey B, Kolliker R (2006) A novel strategy to extract specific phylogenetic sequence information from community T-RFLP. J Microbiol Methods 66:512–520
Zinger L, Lejon DPH, Baptist F, Bouasria A, Aubert S, Geremia RA, Choler P (2011) Contrasting diversity patterns of crenarchaeal, bacterial and fungal soil communities in an Alpine landscape. Plos One 6
Acknowledgements
Financial support for this study was provided by the “Biosphere–geosphere interactions: Linking climate change, weathering, soil formation and ecosystem evolution (BigLink)” project of the Competence Centre Environment and Sustainability (CCES) of the ETH Domain. It was also supported by the Genetic Diversity Centre (GDC) of the ETH Zurich. We thank Martin Hartmann and Yves Wurmitzer for their help with the phylogenetic analysis. We are also grateful to Gerhard Furrer and Michael Plötze for valuable discussions. Additionally, we would like to thank three anonymous reviewers for their valuable input in improving this manuscript. Finally, we thank our linguistic lecturer Silvia Dingwall for correcting and improving the English text.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
Bacterial phylogenetic tree calculated by Bayesian inference using 748 bp, showing affiliation of clones to closest related sequences of different cold and soil habitats and other reference sequences. The spirochete Borrelia burgdorferi (L36160) was chosen as outgroup. Young 2-year-old site clones (named 1_b), intermediate 62-year-old site clones (7_b) and old 110-year-old site clones (18_b) are shown. For clarity, we removed similar sequences in the tree. The accession numbers of the reference sequences are given in brackets. Posterior probabilities from the Bayesian analysis are only shown when below 75% (DOC 42 kb)
Fig. S2
Archaeal phylogenetic tree calculated by Bayesian inference using 570 bp, showing affiliation of clones to closest related sequences of different cold and soil habitats and other reference sequences. The Korarchaeota (AF255604) was chosen as outgroup. Young 2-year-old site clones (named 1_a), intermediate 62-year-old site clones (7_a) and old 110-year-old site clones (18_a) are shown. For clarity, we removed similar sequences in the tree. The accession numbers of the reference sequences are given in brackets. Posterior probabilities from the Bayesian analysis are only shown when below 75% (DOC 49 kb)
Fig. S3
Fungal phylogenetic tree calculated by Bayesian inference using 751bp, showing affiliation of clones to closest related sequences of different cold and soil habitats and other reference sequences. A member of the Chytridiomycota, Cladochytrium sp. (AB586077) was chosen as outgroup. Young 2 year-old site clones (named 1_f); intermediate 62 year-old site clones (7_f) and old 110 year-old site clones (18_f) are shown. For clarity we removed similar sequences in the tree. The accession numbers of the reference sequences are given in brackets. Posterior probabilities from the Bayesian analysis are only shown when below 75%. (DOC 59 kb)
Table S1
Results of sequence analysis of bacteria, archaea and fungi for the young (site 0, 2-year-old soil), intermediate (site 6, 62-year-old soil) and old soil (site 17, 110-year-old soil), calculated with Mothur with a 97% identity for a unique genus. A total of 192 clones were recovered for each phylogenetic group and site. After RFLP pattern analysis for selecting the clones with unique OTUs, 100 bacterial clones, 64 archaeal clones and 50 fungal clones were sequenced (DOC 37 kb)
Rights and permissions
About this article
Cite this article
Zumsteg, A., Luster, J., Göransson, H. et al. Bacterial, Archaeal and Fungal Succession in the Forefield of a Receding Glacier. Microb Ecol 63, 552–564 (2012). https://doi.org/10.1007/s00248-011-9991-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9991-8