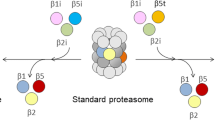
Abstract
The proteasome is the main protein-degrading machine within the cell, producing ligands for MHC class I molecules. It is a cylindrical multicatalytic protease complex, and the catalytic activity is mediated by the three subunits β1, β2, and β5 which possess caspase-, trypsin-, and chymotrypsin-like activities, respectively. By stimulation with interferon (IFN)-γ the replacement of these subunits by β1i, β2i, and β5i is induced leading to formation of immunoproteasomes with altered proteolytic and antigen processing properties. The genes coding for these immunosubunits are restricted to jawed vertebrates but have so far not been found in the genomes of birds, e.g., chicken, turkey, quail, black grouse and zebra finch. However, the chicken genome sequences are not completely assigned; therefore, we investigated the presence of immunoproteasome on protein level. 20S proteasome was purified from the chicken brain, blood, spleen, and bursa of Fabricius, followed by separation via two-dimensional (2D) gel electrophoresis. We analyzed the protein spots derived from the spleen and brain by mass spectrometry and could identify all 14 proteasomal subunits, but there were no differences detectable in the spot patterns. Moreover, we stimulated the chicken spleen cells with phorbol 12-myristate 13-acetate (PMA) and ionomycin aiming at the induction of immunoproteasome, but in spite of the induction of proliferation and IFN-γ, no evidence for immunoproteasome formation in chicken could be obtained. This result was substantiated by the finding that 20S proteasomes isolated from immune and non-immune tissues showed very similar peptidolytic activities. Taken together, our results indicate that chicken lack immunoproteasomes also on protein level.




Similar content being viewed by others
References
Agarwal AK, Xing C, DeMartino GN, Mizrachi D, Hernandez MD, Sousa AB, Martinez de Villarreal L, dos Santos HG, Garg A (2010) PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet 87:866–872
Arima K, Kinoshita A, Mishima H, Kanazawa N, Kaneko T, Mizushima T, Ichinose K, Nakamura H, Tsujino A, Kawakami A, Matsunaka M, Kasagi S, Kawano S, Kumagai S, Ohmura K, Mimori T, Hirano M, Ueno S, Tanaka K, Tanaka M, Toyoshima I, Sugino H, Yamakawa A, Niikawa N, Furukawa F, Murata S, Eguchi K, Ida H, Yoshiura K (2011) Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc Natl Acad Sci U S A 108:14914–14919
Balakrishnan CN, Ekblom R, Volker M, Westerdahl H, Godinez R, Kotkiewicz H, Burt DW, Graves T, Griffin DK, Warren WC, Edwards SV (2010) Gene duplication and fragmentation in the zebra finch major histocompatibility complex. BMC Biol 8:29
Basler M, Groettrup M (2012) Immunoproteasome-specific inhibitors and their application. Methods Mol Biol 832:391–401
Basler M, Dajee M, Moll C, Groettrup M, Kirk CJ (2010) Prevention of experimental colitis by a selective inhibitor of the immunoproteasome. J Immunol 185:634–641
Basler M, Mundt S, Muchamuel T, Moll C, Jiang J, Groettrup M, Kirk CJ (2014) Inhibition of the immunoproteasome ameliorates experimental autoimmune encephalomyelitis. EMBO Mol Med 6:226–238
Chaves LD, Krueth SB, Reed KM (2009) Defining the turkey MHC: sequence and genes of the B locus. J Immunol 183:6530–6537
Claverol S, Burlet-Schiltz O, Girbal-Neuhauser E, Gairin JE, Monsarrat B (2002) Mapping and structural dissection of human 20 S proteasome using proteomic approaches. Mol Cell Proteomics 1:567–578
Froment C, Uttenweiler-Joseph S, Bousquet-Dubouch MP, Matondo M, Borges JP, Esmenjaud C, Lacroix C, Monsarrat B, Burlet-Schiltz O (2005) A quantitative proteomic approach using two-dimensional gel electrophoresis and isotope-coded affinity tag labeling for studying human 20S proteasome heterogeneity. Proteomics 5:2351–2363
Groettrup M, Ruppert T, Kuehn L, Seeger M, Standera S, Koszinowski U, Kloetzel PM (1995) The interferon-gamma-inducible 11 S regulator (PA28) and the LMP2/LMP7 subunits govern the peptide production by the 20 S proteasome in vitro. J Biol Chem 270:23808–23815
Groettrup M, Kraft R, Kostka S, Standera S, Stohwasser R, Kloetzel PM (1996) A third interferon-gamma-induced subunit exchange in the 20S proteasome. Eur J Immunol 26:863–869
Hayter JR, Doherty MK, Whitehead C, McCormack H, Gaskell SJ, Beynon RJ (2005) The subunit structure and dynamics of the 20S proteasome in chicken skeletal muscle. Mol Cell Proteomics 4:1370–1381
Hershko A, Ciechanover A, Varshavsky A (2000) Basic Medical Research Award. The ubiquitin system. Nat Med 6:1073–1081
Huang Y, Li Y, Burt DW, Chen H, Zhang Y, Qian W, Kim H, Gan S, Zhao Y, Li J, Yi K, Feng H, Zhu P, Li B, Liu Q, Fairley S, Magor KE, Du Z, Hu X, Goodman L, Tafer H, Vignal A, Lee T, Kim KW, Sheng Z, An Y, Searle S, Herrero J, Groenen MA, Crooijmans RP, Faraut T, Cai Q, Webster RG, Aldridge JR, Warren WC, Bartschat S, Kehr S, Marz M, Stadler PF, Smith J, Kraus RH, Ren L, Fei J, Morisson M, Kaiser P, Griffin DK, Rao M, Pitel F, Wang J, Li N (2013) The duck genome and transcriptome provide insight into an avian influenza virus reservoir species. Nat Genet 45:776–783
Huber EM, Basler M, Schwab R, Heinemeyer W, Kirk CJ, Groettrup M, Groll M (2012) Immuno- and constitutive proteasome crystal structures reveal differences in substrate and inhibitor specificity. Cell 148:727–738
Ichikawa HT, Conley T, Muchamuel T, Jiang J, Lee S, Owen T, Barnard J, Nevarez S, Goldman BI, Kirk CJ, Looney RJ, Anolik JH (2012) Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis Rheum 64:493–503
Kalim KW, Basler M, Kirk CJ, Groettrup M (2012) Immunoproteasome subunit LMP7 deficiency and inhibition suppresses Th1 and Th17 but enhances regulatory T cell differentiation. J Immunol 189:4182–4193
Kaufman J (2008) The Avian MHC. In: Davison F, Kaspers B, Schat KA (eds) Avian immunology, 1st edn. Elsevier Ltd., London, pp 159–181
Kaufman J, Volk H, Wallny HJ (1995) A “minimal essential Mhc” and an “unrecognized Mhc”: two extremes in selection for polymorphism. Immunol Rev 143:63–88
Kaufman J, Milne S, Gobel TW, Walker BA, Jacob JP, Auffray C, Zoorob R, Beck S (1999) The chicken B locus is a minimal essential major histocompatibility complex. Nature 401:923–925
Khan S, van den Broek M, Schwarz K, de Giuli R, Diener PA, Groettrup M (2001) Immunoproteasomes largely replace constitutive proteasomes during an antiviral and antibacterial immune response in the liver. J Immunol 167:6859–6868
Kitamura A, Maekawa Y, Uehara H, Izumi K, Kawachi I, Nishizawa M, Toyoshima Y, Takahashi H, Standley DM, Tanaka K, Hamazaki J, Murata S, Obara K, Toyoshima I, Yasutomo K (2011) A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest 121:4150–4160
Kniepert A, Groettrup M (2014) The unique functions of tissue-specific proteasomes. Trends Biochem Sci 39:17–24
Koch M, Camp S, Collen T, Avila D, Salomonsen J, Wallny HJ, van Hateren A, Hunt L, Jacob JP, Johnston F, Marston DA, Shaw I, Dunbar PR, Cerundolo V, Jones EY, Kaufman J (2007) Structures of an MHC class I molecule from B21 chickens illustrate promiscuous peptide binding. Immunity 27:885–899
Kremer M, Henn A, Kolb C, Basler M, Moebius J, Guillaume B, Leist M, Van den Eynde BJ, Groettrup M (2010) Reduced immunoproteasome formation and accumulation of immunoproteasomal precursors in the brains of lymphocytic choriomeningitis virus-infected mice. J Immunol 185:5549–5560
Miteva M, Keusekotten K, Hofmann K, Praefcke GJ, Dohmen RJ (2010) Sumoylation as a signal for polyubiquitylation and proteasomal degradation. Subcell Biochem 54:195–214
Moebius J, van den Broek M, Groettrup M, Basler M (2010) Immunoproteasomes are essential for survival and expansion of T cells in virus-infected mice. Eur J Immunol 40:3439–3449
Moiseeva TN, Bottrill A, Melino G, Barlev NA (2013) DNA damage-induced ubiquitylation of proteasome controls its proteolytic activity. Oncotarget 4:1338–1348
Morera D, Roher N, Ribas L, Balasch JC, Donate C, Callol A, Boltana S, Roberts S, Goetz G, Goetz FW, MacKenzie SA (2011) RNA-Seq reveals an integrated immune response in nucleated erythrocytes. PLoS One 6:e26998
Muchamuel T, Basler M, Aujay MA, Suzuki E, Kalim KW, Lauer C, Sylvain C, Ring ER, Shields J, Jiang J, Shwonek P, Parlati F, Demo SD, Bennett MK, Kirk CJ, Groettrup M (2009) A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med 15:781–787
Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, Takahama Y, Tanaka K (2007) Regulation of CD8+ T cell development by thymus-specific proteasomes. Science 316:1349–1353
Mustonen L, Alinikula J, Lassila O, Nera K-P (2001) Bursa of Fabricius. eLS. John Wiley & Sons, Ltd
Nitta T, Murata S, Sasaki K, Fujii H, Ripen AM, Ishimaru N, Koyasu S, Tanaka K, Takahama Y (2010) Thymoproteasome shapes immunocompetent repertoire of CD8+ T cells. Immunity 32:29–40
Rodriguez-Mendez AJ, Luna-Acosta JL, Carranza M, Harvey S, Aramburo C, Luna M (2010) Growth hormone expression in stromal and non-stromal cells in the bursa of Fabricius during bursal development and involution: causal relationships? Gen Comp Endocrinol 167:297–307
Salter RD, Cresswell P (1986) Impaired assembly and transport of HLA-A and -B antigens in a mutant TxB cell hybrid. EMBO J 5:943–949
Schmidt F, Dahlmann B, Janek K, Kloss A, Wacker M, Ackermann R, Thiede B, Jungblut PR (2006) Comprehensive quantitative proteome analysis of 20S proteasome subtypes from rat liver by isotope coded affinity tag and 2-D gel-based approaches. Proteomics 6:4622–4632
Schmidtke G, Aichem A, Groettrup M (2014) FAT10ylation as a signal for proteasomal degradation. Biochim Biophys Acta 1843:97–102
Shiina T, Shimizu S, Hosomichi K, Kohara S, Watanabe S, Hanzawa K, Beck S, Kulski JK, Inoko H (2004) Comparative genomic analysis of two avian (quail and chicken) MHC regions. J Immunol 172:6751–6763
Shiina T, Briles WE, Goto RM, Hosomichi K, Yanagiya K, Shimizu S, Inoko H, Miller MM (2007) Extended gene map reveals tripartite motif, C-type lectin, and Ig superfamily type genes within a subregion of the chicken MHC-B affecting infectious disease. J Immunol 178:7162–7172
St Paul M, Barjesteh N, Paolucci S, Pei Y, Sharif S (2012a) Toll-like receptor ligands induce the expression of interferon-gamma and interleukin-17 in chicken CD4+ T cells. BMC Res Notes 5:616
St Paul M, Paolucci S, Barjesteh N, Wood RD, Schat KA, Sharif S (2012b) Characterization of chicken thrombocyte responses to Toll-like receptor ligands. PLoS One 7:e43381
St Paul M, Paolucci S, Read LR, Sharif S (2012c) Characterization of responses elicited by Toll-like receptor agonists in cells of the bursa of Fabricius in chickens. Vet Immunol Immunopathol 149:237–244
Sutoh Y, Kondo M, Ohta Y, Ota T, Tomaru U, Flajnik MF, Kasahara M (2012) Comparative genomic analysis of the proteasome beta5t subunit gene: implications for the origin and evolution of thymoproteasomes. Immunogenetics 64:49–58
Wallny HJ, Avila D, Hunt LG, Powell TJ, Riegert P, Salomonsen J, Skjodt K, Vainio O, Vilbois F, Wiles MV, Kaufman J (2006) Peptide motifs of the single dominantly expressed class I molecule explain the striking MHC-determined response to Rous sarcoma virus in chickens. Proc Natl Acad Sci U S A 103:1434–1439
Wang B, Ekblom R, Strand TM, Portela-Bens S, Hoglund J (2012) Sequencing of the core MHC region of black grouse (Tetrao tetrix) and comparative genomics of the galliform MHC. BMC Genomics 13:553
Wu Z, Kaiser P (2011) Antigen presenting cells in a non-mammalian model system, the chicken. Immunobiology 216:1177–1183
Acknowledgments
We thank Gerardo Alvarez Salinas, Gerhard Gröttrup, and Lars Krieger for their help with proteasome purification and Thomas Göbel (LMU Munich) and Jim Kaufman (Cambridge University) for helpful advice concerning the spleen stimulation experiments. We are grateful to Julia Koerner and Michael Basler for help with flow cytometric analyses. The team of the animal research facility of Konstanz University is acknowledged for assistance during animal experimentation. This work was supported by a grant from the German Research Foundation (Nr. GR 1517/14-1) and a grant from the Swiss National Science Foundation (Nr. 31003A_138451).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erath, S., Groettrup, M. No evidence for immunoproteasomes in chicken lymphoid organs and activated lymphocytes. Immunogenetics 67, 51–60 (2015). https://doi.org/10.1007/s00251-014-0814-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-014-0814-1