Abstract
The polymorphism of immunogenes of the major histocompatibility complex (MHC) is thought to influence the functional plasticity of immune responses and, consequently, the fitness of populations facing heterogeneous pathogenic pressures. Here, we evaluated MHC variation (allelic richness and divergence) and patterns of selection acting on the two highly polymorphic MHC class II loci (DRB and DQB) in the endangered primate Madame Berthe’s mouse lemur (Microcebus berthae). Using 454 pyrosequencing, we examined MHC variation in a total of 100 individuals sampled over 9 years in Kirindy Forest, Western Madagascar, and compared our findings with data obtained previously for its sympatric congener, the grey mouse lemur (Microcebus murinus). These species exhibit a contrasting ecology and demography that were expected to affect MHC variation and molecular signatures of selection. We found a lower allelic richness concordant with its low population density, but a similar level of allelic divergence and signals of historical selection in the rare feeding specialist M. berthae compared to the widespread generalist M. murinus. These findings suggest that demographic factors may exert a stronger influence than pathogen-driven selection on current levels of allelic richness in M. berthae. Despite a high sequence similarity between the two congeners, contrasting selection patterns detected at DQB suggest its potential functional divergence. This study represents a first step toward unravelling factors influencing the adaptive divergence of MHC genes between closely related but ecologically differentiated sympatric lemurs and opens new questions regarding potential functional discrepancy that would explain contrasting selection patterns detected at DQB.
Similar content being viewed by others
Introduction
The island of Madagascar, one of the world’s biodiversity hotspots, has faced rapid deforestation over the last century, resulting in population fragmentation of many endemic primates (Lemuriformes) (Ganzhorn et al. 2001; Mittermeier et al. 1992; Schwitzer et al. 2013a, b), 94 % of which are currently classified as threatened (Schwitzer et al. 2013a). Many of these species are highly arboreal, inhabit restricted biogeographic ranges and exhibit fast life histories and higher population turnover compared to most anthropoid primates; however, the average life span is thought to be compromised by high extrinsic mortality pressure in the wild populations (Kraus et al. 2008; Fichtel 2012; Kappeler 2012). These characteristics make lemurs particularly vulnerable to an ongoing habitat degradation that may disrupt gene flow and cause local demographic fluctuations. This may in turn result in an irreversible loss of genetic diversity in lemurs, especially those species with restricted spatial distribution. Given the ongoing rate of deforestation, rapid surveys of remaining genetic diversity and its potential consequences for the future viability of these populations are essential to determine conservation priorities (Kremen et al. 2008). Although difficult to detect in small populations (Chikhi et al. 2010), signals of demographic or genetic bottlenecks or decreasing genetic diversity at neutral markers seem present across lemur taxa (Louis et al. 2005; Olivieri et al. 2008; Markolf et al. 2008; Radespiel et al. 2008; Craul et al. 2009; Razakamaharavo et al. 2010; Holmes et al. 2013; Baden et al. 2014). However, little is known regarding its potential fitness consequences and the capacity of such populations to maintain an effective level of functional (adaptive) genetic variation (allelic richness and divergence), sufficient to ensure their health and survival.
Genes of the major histocompatibility complex (MHC) are well suited to study the adaptive maintenance of genetic variation given their immune function (Klein 1986) and extreme polymorphism (e.g. Hedrick 2002; Garrigan and Hedrick 2003; Sommer 2005; Piertney and Oliver 2006; Spurgin and Richardson 2010). MHC genes are coding for MHC molecules (cell surface glycoproteins) that trigger the immune response by presenting antigens at antigen-binding sites (ABS) to T-lymphocytes, which then activate further components of the immune system. Each MHC molecule can bind only a limited array of antigens, and a number of mechanisms may increase the spectrum of antigens recognised, including gene duplications, extensive allelic diversity, polymorphism at ABS (Hughes and Nei 1988), or polymorphism outside ABS that may alter the 3D positioning of ABS contact residues (Bjorkman and Burmeister 1994).
Balancing selection, primarily exerted by pathogen pressure, is thought to represent one of the major forces driving MHC polymorphism (reviewed in Bernatchez and Landry 2003; Sommer 2005; Piertney and Oliver 2006; Milinski 2006; Spurgin and Richardson 2010). Three non-mutually exclusive evolutionary mechanisms have been proposed to explain this polymorphism. First, due to the codominant expression of MHC alleles and the function of MHC molecules in the immune response, MHC heterozygous individuals may be at an advantage in a population facing heterogeneous pathogen pressures (i.e. heterozygote advantage; Doherty and Zinkernagel 1975). Second, MHC alleles that are advantageous in protection against dominant pathogens in a given environment (Apanius et al. 1997) may temporarily rise in frequency, until pathogens evolve resistance to the most common host alleles, which are then progressively replaced by rarer alleles (i.e. frequency-dependent selection; Snell 1968; Bodmer 1972; Borghans et al. 2004). Finally, parasite communities typically vary in space and time and may thereby select distinct sets of MHC alleles in host populations (i.e. fluctuating selection; Hedrick 1999; Spurgin and Richardson 2010; Eizaguirre et al. 2009, 2012). As a result, patterns of selection on MHC genes are likely to be durably affected by the diversity and frequency of MHC alleles present in the host population and, hence, by its demography (Hedrick 1972; Borghans et al. 2004). The interruption of gene flow across fragmented populations and the reduction of effective population sizes can therefore result in a loss of genetic diversity through genetic drift and inbreeding (Wright 1969; Keller and Waller 2002; Frankham et al. 2002) and may disrupt balancing selection (Hughes and Yeager 1998) that might consequently compromise population capacity to respond to changing pathogenic pressures (O’Brien and Evermann 1988).
In support of this assumption, a possible link between highly divergent or specific MHC genotypes and individual fitness (Schad et al. 2005; Schwensow et al. 2007, 2010a, b; Sommer et al. 2014) and mate choice (Schwensow et al. 2008a, b; Huchard et al. 2013) has been suggested for two widely distributed lemurs—Microcebus murinus and Cheirogaleus medius (Cheirogaleidae, Primates). Consequently, the evaluation of MHC variation retained in populations of endangered confamiliar species can provide valuable insights to assess their viability. The complexity of MHC genotyping has long impaired detailed genetic studies of free-ranging species with unknown genomic organisation. Next-generation sequencing (NGS) technologies promise progress in this area by overcoming some of the technical difficulties associated with the complexity of MHC genotyping and by allowing cost-effective processing of large datasets (reviewed in Babik 2010; Huchard and Pechouskova 2014; Koboldt et al. 2013; Lighten et al. 2014a).
Here, we investigated MHC variation (allelic richness and divergence) and patterns of selection of two highly polymorphic MHC class II genes, DRB and DQB, in the endangered Madame Berthe’s mouse lemur, Microcebus berthae (B1ab.i-iii, Andriaholinirina et al. 2014), by genotyping a total of 100 individuals sampled over 9 years in three study areas. This world’s smallest primate (ca. 30 g) is endemic to the dry forests of the Menabe region in western Madagascar (Schmid and Kappeler 1994; Ganzhorn et al. 2001; Schäffler and Kappeler 2014), which has recently been identified as a priority site for conservation (Schwitzer et al. 2013a). The distribution of M. berthae is restricted to an area of less than 810 km2 within two forest fragments and a narrow corridor connecting them (e.g. Rasoloarison et al. 2000; Schäffler and Kappeler 2014). Its population density varies across its geographic range (30–100 individuals/km2) and seems to be affected by habitat heterogeneity and anthropogenic disturbances (Schwab and Ganzhorn 2004; Schäffler and Kappeler 2014).
Next, we compared our findings with data obtained across a similar temporal scale from a population of M. murinus, a sympatric congener (e.g. Weisrock et al. 2010), which in comparison to M. berthae presents several key ecological and demographic differences. M. berthae is a feeding specialist relying mostly on dispersed fast depleting resources, such as homopteran secretions or arthropods. This feeding strategy is thought to promote an intense scramble competition leading to spatial avoidance, overdispersion and lower rate of social interactions among conspecifics (Dammhahn and Kappeler 2009). In contrast, the more opportunistic feeding niche of M. murinus, including diverse plant and animal matter, seems to reduce competition and facilitate spatial proximity and social interactions among conspecifics (Dammhahn and Kappeler 2008a, b, 2010). The ecological flexibility of M. murinus is also reflected by its wide distribution across southern and western Madagascar. Its population density is higher and population size larger in Kirindy Forest than those of M. berthae (Eberle and Kappeler 2004; Dammhahn and Kappeler 2005, 2008b). We expect these inter-specific contrasts to influence MHC variation. First, given that allelic richness appears to be a function of effective population size (Hedrick 1985), we expect MHC allelic richness of M. berthae to be lower than in M. murinus. Second, the larger population of M. murinus might harbour a more diverse array of pathogens (Anderson and May 1978; Nunn et al. 2003; Hughes and Page 2007; see also Altizer et al. 2007), and this effect might be enhanced by a broader feeding niche and more frequent encounters with conspecifics offering a greater chance of pathogen encounter and transmission. In contrast, low population densities and low rates of social interactions in combination with a narrow feeding niche could result in relaxed pathogen-mediated pressure in M. berthae. As such, we predict to detect weaker tracks of pathogen-driven selection on M. berthae MHC alleles compared to M. murinus.
Methods
Sample collection and DNA extraction
DNA samples were collected from M. berthae from three sub-populations captured between 2005 and 2013 using baited Sherman life traps set within 25-ha study areas (N5, CS7 and Savannah) located within a 12.500-ha concession of Kirindy Forest of the Centre National de Formation, d’Etude et de Recherche en Environnement et Foresterie (CNFEREF) de Morondava (Madagascar: 44° 39′ E, 20° 03′ S, Kappeler and Fichtel 2012). The centres of the study areas N5-CS7 and Savannah-CS7 are situated ca. 2–2.5 km and N5-Savannah ca. 4–4.5 km away from each other, respectively. In total, we collected samples from 100 individuals, with sample sizes reflecting contrasting population densities and sampling effort across the years at each study area (Electronic supplementary material ESM 1; N5: n = 80; 42♂/37♀/1 n.a.; CS7: n = 14, 1♂/13 n.a.; Savannah: n = 6, 3♀/3♂). At first capture, each individual was briefly immobilised with 10 μl Ketanest 100 (s.c.) (Rensing 1999), individually marked with a sub-dermal microtransponder (Trovan, Usling, Germany) for other studies (e.g. Dammhahn and Kappeler 2005, 2008a, b, 2010), and a small ear biopsy of 2–3 mm2 was taken and preserved in 70 % ethanol. Genomic DNA was extracted from ear biopsies following standard protocol (Qiagen QIAmp DNA Mini-Kit, Qiagen Germany). We have adhered to the Guidelines for the Treatment of Animals in Behavioural Research and Teaching (Animal Behaviour 2006, 71: 245-253) and the legal requirements of the country (Madagascar) in which the fieldwork was carried out.
454 library preparation
PCR amplification targeting the two loci of the most variable parts of the MHC class II region, DRB and DQB, was performed using primers that flank the functionally important ABS and captured the full variability in the congener M. murinus (Schad et al. 2004; Averdam et al. 2011). PCR reaction mix and amplification conditions are summarised in ESM 2. Each individual PCR product (further referred to as amplicon) was electrophoresed on 1 % agarose gel to verify successful amplification. Primer design and the preparation of locus-specific amplicon libraries were described elsewhere (Huchard et al. 2012). Sequencing was conducted according to standard protocols for GS Junior sequencing (Roche, 454 pyrosequencing). All sequencing reads retrieved from a total of six sequencing runs were processed according to a post-sequencing quality control procedure following Huchard et al. (2012).
454 library processing
Allelic discrimination and evaluation of the number of loci
Artefactual alleles introduced by PCR or sequencing errors and assessing the sequencing depth necessary for reliable genotyping are well-known technical challenges associated with NGS that might compromise the reliability of assessments of MHC polymorphism (reviewed in Babik 2010; Lighten et al. 2014a).
Here, we adjusted some of the filtering steps proposed by previous authors (e.g. Babik et al. 2009; Galan et al. 2010; Zagalska-Neubauer et al. 2010; Huchard et al. 2012) to discriminate true versus artefactual alleles, relying on two central assumptions: (1) Artefactual alleles should show high similarity to one of the two parental sequences they originated from within amplicons, either by single point mutation or indels causing a shift in the reading frame, or by recombination of the two parental sequences (chimeras), and (2) artefactual alleles should be relatively rare, compared to true alleles, both across and within amplicons. In contrast to studies mentioned above, we did not identify artefactual alleles based on global allelic frequency thresholds established across amplicons but evaluated allele status both across and within each amplicon.
Both manual alignment (Multalin; Corpet 1988) and two numeric indices of allelic frequency were used to critically evaluate a potential number of loci and to discriminate true alleles from artefacts: (1) the mean per amplicon frequency (MPAF) of any given allele as the proportion of reads from an amplicon assigned to this allele, averaged across all amplicons possessing this allele, and (2) the relative per amplicon frequency (RPAF) of each allele as the proportion of reads retrieved for each given allele within a given amplicon. We predicted that artefactual alleles should be relatively rare, compared to true alleles across amplicons (reflected by low MPAF) and within amplicons (reflected by low RPAF). While MPAF can help to identify artefacts returned at low frequencies across sequencing runs, RPAF can help to identify heterogeneities in the distribution of within-amplicon allelic frequency. These can be either artefactual alleles that occur non-randomly across sequencing runs or at high frequencies only in few amplicons, skewing their MPAF (i.e. run-specific sequencing errors or homopolymers) (see also Lenz and Becker 2008; Harismendy et al. 2009; Gilles et al. 2011; Sommer et al. 2013; Lighten et al. 2014a, b), or cross-amplicon contaminations of true alleles (with high MPAF and low RPAF). The occurrence of such DNA carryover contaminants has recently been acknowledged as an underrated source of genotyping errors associated with NGS (Huchard et al. 2012; Li and Stoneking 2012; Sommer et al. 2013; Lighten et al. 2014a, b). Here, we did not systematically eliminate these suspected contaminants by using a fixed threshold for a minimum frequency per amplicon (here referred to as RPAF) under which alleles are filtered out within amplicons (e.g. Sepil et al. 2012; Huchard et al. 2012), to avoid eliminating true alleles and thereby generating potential allelic dropout (van Oosterhout et al. 2006; Sommer et al. 2013). Rather, the status of amplicons suspected of contaminations by true alleles was clarified by replicating affected amplicons.
In the first step of the allele sorting procedure, we attempted to evaluate whether target genes may be duplicated in order to assess the sequencing depth required to ensure reliable genotyping. Although there was no indication of a loci duplication in M. murinus (Averdam et al. 2011; Huchard et al. 2012), we could not assume the same in M. berthae due to extensive variation in the genomic organisation of MHC within and across species (e.g. Kelley et al. 2005; Winternitz and Wares 2013; Lighten et al. 2014b). Therefore, we investigated the MPAF and RPAF distribution of the most common to the least common alleles across all amplicons, assuming that in the case of non-duplication, we would detect a notable drop between the two most common and remaining alleles (see also Babik et al. 2009; Huchard et al. 2012). Next, we evaluated our findings by manual alignment of all alleles within each amplicon and attempted to discriminate artefactual from true alleles based on our assumptions—similarity to parental allele and low MPAF and RPAF. When the status of alleles remained ambiguous, affected amplicons were replicated. Finally, we replicated those few amplicons that passed allele sorting with more true alleles than expected given the estimated number of loci to check whether this came from the genuine locus duplication or from an artefact.
Assessment of minimum sequencing depth and genotyping reliability
Based on the estimated number of loci, we proceeded with a final screening step to evaluate the sequencing depth necessary for accurate genotyping using the program ‘Negative Multinomial’ (http://www.lirmm.fr/~caraux/Bioinformatics/NegativeMultinomial/) developed by Galan et al. (2010). Amplicons that did not return a sufficient amount of reads based on this estimate were re-genotyped.
The efficiency of allele-sorting was then evaluated through two steps. First, assuming that artefacts originated during a single PCR reaction or introduced by a genotyping mistake would not occur independently in many amplicons, we investigated the relationship between the MPAF of each allele and the number of amplicons possessing this allele. We then correlated both values before and after allele sorting. Here, we expected a positive relationship before allele sorting—due to the fact that artefacts should display both low MPAF and be retrieved in few individuals only—that would disappear after allele sorting (Babik et al. 2009; Huchard et al. 2012). Second, a set of amplicons were additionally replicated for each loci within independent sequencing runs (DRB n = 23; DQB n = 27) to assess the reliability of our genotyping for each locus within.
Sequence analysis and phylogeny reconstruction
All true alleles were retained for downstream analysis, including those that were possessed by one individual only to prevent the elimination of rare alleles leading to an underestimation of allelic richness and to avoid generating false homozygotes. However, these alleles were not submitted in public repositories.
Allelic divergence was evaluated by computing average pairwise distances (number of differences) among all pairs of nucleotide and amino acid sequences in each locus in MEGA 6 (Tamura et al. 2013). Evolutionary relationships between amino acid sequences of both loci found by this study in M. berthae and previously in M. murinus were constructed in MEGA, using a neighbour-joining algorithm with Poisson correction (Saitou and Nei 1987; Zuckerkandl and Pauling 1965). Mimu sequences originated from 664 individuals captured within the study area CS7 between the years 2000 and 2010. These Mimu alleles were retrieved using 454 technology and comparable allele validation steps (see Huchard et al. 2012). The repeatability of sequence alignment was determined by a bootstrap analysis with 1000 replications.
Allelic richness of both loci detected within the study population of M. berthae was compared to those previously described for M. murinus (Huchard et al. 2012). To evaluate the number of alleles detected for a given sampling effort, we conducted a permutation test in R (www.r-project.org). Here, we randomly selected 10 individuals and counted the number of distinct alleles detected. This procedure was repeated 100 times to calculate a mean and SD for each sampling effort, adding 10 individuals at each step until 100. This procedure was conducted for both M. berthae and M. murinus and plotted for both loci to illustrate the number of distinct alleles detected per given sampling effort.
Population genetic analysis
Linkage disequilibrium between DRB and DQB loci was tested using a likelihood ratio test, where the null hypothesis of no association between loci (linkage equilibrium) is compared to the hypothesis of a possible association (Slatkin and Excoffier 1996). The significance of the procedure is found by computing the null distribution of this ratio under the hypothesis of linkage equilibrium using 10,000 permutations implemented in Arlequin 3.5.1.3. (Excoffier and Lischer 2010). Using GENEPOP v 1.2 (Rousset and Raymond 1995), a null allelic frequency was estimated by maximum likelihood estimation (EM algorithm; Dempster et al. 1977), and deviations from Hardy-Weinberg equilibrium (HWE) were calculated for each locus separately using the exact U-score test (Rousset and Raymond 1995), with the alternative hypothesis predicting heterozygote excess. The extent of genetic differentiation among sub-populations (regardless of sex and year cohort) was examined by pairwise F ST at both loci using Arlequin (10,000 permutations, Wright 1965). Comparisons between adult males and females and across year cohorts were not included due to uneven and small sample sizes (see above and ESM 1).
Test of positive selection
The presence of positively selected sites (PSS) was investigated in both genes separately. PSS are characterised by ω > 1 with ω = d N /d S , and d N and d S being the relative amounts of substitutions at non-silent (d N ) and silent (d S ) codon sites. First, we investigated the strength of positive selection using the likelihood ratio modelling approach. We compared two models of the codon evolution: the null model, where ω < 1 and varies according to the beta distribution (model M7), and a model allowing an additional class of sites, where ω > 1, to account for a possible occurrence of PSS (model M8) using a likelihood ratio test (LRT) (in Yang et al. 2000). If model M8 fits the data better than M7, PSS were identified through Bayes Empirical Bayes (BEB) procedure and retained for evaluation by the next step if statistically significant (Yang et al. 2005), using the package CodeML implemented in PAML 4.7 (Yang 2007).
As a second approach, we estimated values of d N and d S and their standard errors by calculating the pairwise number of silent and non-silent substitutions (Nei and Gojobori 1986) applying Jukes-Cantor correction for multiple hits implemented in MEGA. This rather conservative approach considers all possible evolutionary pathways (excluding termination codons) leading from one codon to another as equally probable and is thereby expected to provide conservative (minimum) estimates of numbers of substitutions compared to the positive selection hypothesis ω > 1 (Nei and Kumar 2000). A codon-based Z-test of selection was performed to test whether both PSS (ω > 1) and non-PSS (ω < 1), identified by the previous approach using BEB procedure, were under positive selection. Furthermore, we compared ω in ABS and non-ABS. Their location was derived from referential human sequences (HLA-DQB, HLA-DRB; Bondinas et al. 2007) and compared with previously detected PSS and non-PSS. Finally, overall values of d N and d S were calculated.
Results
Allelic discrimination and evaluation of the number of loci
A total of 321 unique sequences were retrieved for DRB from 148 amplicons (including 49 replicates, see below) and 105 sequences for DQB from 130 amplicons (including 32 replicates), in the range of 162–170 bp (excluding primers). For more details and statistics of sequencing outcome, see ESM 3. The distribution of MPAF and RPAF from the first to sixth most common alleles averaged across all amplicons revealed a notable drop of frequency between the two most common and remaining alleles, suggesting no duplication of either locus (ESM 4).
Within 321 DRB sequences, 286 (89 %) displayed MPAF < 0.05 and were identified by manual alignment as artefacts (95 %) or contaminants (5 %), following our criteria. From the remaining 35 (11 %) alleles with MPAF ≥ 0.05, 13 alleles were identified as artefacts. Additionally, five alleles occurring within a single amplicon (MPAF 0.05–0.08) were identified as contaminants and discarded (see below). The remaining 17 alleles (MPAF 0.08–0.69) were retained as true alleles. In DQB, 74 (70 %) of all 105 sequences displayed MPAF < 0.05, and all of those were eliminated as artefacts (95 %) or contaminants (5 %). Among 31 remaining sequences with MPAF ≥ 0.05, four more sequences were eliminated as artefacts and five as contaminants (MPAF 0.06–0.24). The remaining 22 sequences were retained as true alleles. In both loci, artefacts with MPAF ≥ 0.05 mostly represented homopolymer indels—occurring either at inconsistent frequencies across sequencing runs, within a single sequencing run or amplicon—and their elimination was further supported by running replicates of affected amplicons. Nine out of 14 contaminants were eliminated by running replicates and three in DRB and two in DQB occurred within one amplicon that was due to extensive inflation of artefacts, and thereby inconclusive genotype, excluded in each locus. Overall, 304 (95 %) sequences in DRB and 83 (79 %) in DQB were eliminated. After allele sorting, only three (out of 96 individuals) and six (out of 98 individuals) amplicons had more than two true alleles in DRB and DQB, respectively. In all of them, the existence of a third and in one case fourth allele could be excluded by re-sequencing affected amplicons, which further confirmed our assumption that both loci were non-duplicated.
Assessment of minimum sequencing depth and genotyping reliability
Based on our conclusions of no loci duplication, we used the probabilistic model (Galan et al. 2010) and estimated that a minimum of 18 reads were required for reliable genotyping of each given locus with a confidence level of 0.95. All amplicons with <18 reads (DRB 26; DQB 5) were re-genotyped and replaced in the dataset, except of three amplicons in DRB that did not return >18 reads in the second genotyping attempt. Additionally, 23 amplicons for DRB and 27 for DQB were genotyped in replicates to estimate genotyping reliability, and all of them showed a perfect reproducibility of assigned genotypes.
The correlation between the MPAF of each allele and the number of amplicons possessing this allele was significant before allele sorting in both loci (Pearson’s correlation, DRB n = 321 alleles, r = 0.78, P < 10−15; DQB n = 105 alleles, r = 0.69, P < 10−15), largely driven by the presence of alleles that had both low MPAF and low frequency (ESM 5). This correlation disappeared in DQB after discarding artefacts (Pearson’s correlation n = 22 alleles, r = 0.22, P = 0.33) and was weakened in DRB, though still significant (Pearson’s correlation, DRB n = 17 alleles, r = 0.64, P < 0.006).
Sequence analysis and phylogeny reconstruction
From the total of 17 Mibe-DRB and 22 Mibe-DQB sequences found in 96 and 98 individuals of M. berthae, respectively, none have been described previously. Accession numbers of these alleles as well as the full nucleotide sequence of alleles occurring in only one individual are given in Appendix (Table 3).
Average nucleotide divergence (number of differences) between sequences was comparably high in both loci (mean ± SD; DRB 17.07 ± 2.44; DQB 14.44 ± 2.16). Among Mibe-DRB sequences, we found 47 (29 %) variable nucleotide sites and 35 (21 %) among Mibe-DQB sequences. Each nucleotide sequence of both loci translated into a unique amino acid sequence and the absence of stop codons suggests that all sequences can encode functional proteins. Amino acid sequences revealed 23 (43 %; DRB) and 23 (41 %; DQB) variable sites out of 54 and 56 sites (see below), and comparable amino acid divergence was found in both loci (mean ± SD; DRB 11.73 ± 2.0, DQB 10.76 ± 1.82).
The reconstruction of evolutionary relationships between amino acid sequences of both species revealed two distinct loci-specific clusters, with the exception of three Mibe-DQB sequences (Mibe-DQB*017, *016 and *007), that clustered separately among DRB sequences of both M. berthae and M. murinus (Fig. 1). These three Mibe sequences were retrieved independently at least in two and up to 11 individual amplicons across different sequencing runs, and their presence and loci identity were confirmed by replicating from one to three amplicons possessing affected sequences. Moreover, these three sequences clustered with other DQB sequences when including only Mibe sequences in the analysis (data not shown). There was no clear separation between sequences of the two species in either locus (Fig. 1), and the level of amino acid divergence was comparable within (see above and Huchard et al. 2012) and between allelic pools of the two species (Mimu vs. Mibe-DRB 11.6 ± 2.1; DQB 11.3 ± 1.9). Additionally, an insertion (two codons) causing fragment length polymorphism in DQB, homologous to the one previously described in 19 Mimu-DQB sequences (Huchard et al. 2012), was detected in seven Mibe-DQB sequences. This insertion did not result in a shift of the reading frame, and there was no evidence for stop codons that would indicate a loss of function. These sequences created large distinct clusters, except for one Mibe sequence (Mibe-DQB*008, Fig. 1).
Evolutionary relationships between amino acid sequences for 17 Mibe-DRB (black circles) and 22 Mibe-DQB sequences (grey circles for sequences without 6-bp insertion and red circles for sequences with the insertion) described in this study, including 59 Mimu-DRB (black triangles) and 58 Mimu-DQB sequences (grey triangles for sequences without two-codon insertion and red triangles for sequences with it) described in Huchard et al. (2012). The tree configuration was derived using neighbour-joining algorithm (Bootstrap 1000; Poisson correction) in MEGA 6. Only bootstrap values exceeding 50 % are shown. Accession numbers and nucleotide sequences of M. berthae are presented in Appendix (Table 3) (colour figure online)
Allelic richness (number of distinct alleles) estimated for a given sampling effort (number of sampled individuals) through re-sampling procedure in M. berthae and M. murinus is shown in Fig. 2. The estimated mean of distinct alleles detected per sampling effort is lower in M. berthae in both loci, indicating lower overall allelic richness for a given sampling effort in this study population.
Estimation of allelic richness for a given sampling effort through re-sampling procedure, showing the number of distinct alleles detected when randomly drawing an increasing number of individuals from our sample in M. berthae (red) and M. murinus (blue). Given the similarity of the observed pattern between DRB and DQB, only the plot for DQB loci is shown. The dotted lines indicate the standard deviation around the estimated mean (solid line) (colour figure online)
Population genetic analysis
The null hypothesis of linkage equilibrium between loci could be rejected (χ 2 = 810.33, df = 396, P < 10−06). Allelic distribution patterns were relatively similar in both genes across all sub-populations with allelic frequencies varying widely within each locus from 1 to 33 % in DRB and 1 to 27 % in DQB (Fig. 3).
The estimated frequency of null alleles was low in both loci (<0.01). The comparable observed and expected level of heterozygosity could be found at both loci across all sub-populations (DRB, H O = 0.91, H E = 0.90; DQB, H O and H E = 0.92), and heterozygote excess was not detected in either locus (DRB, FisW&C = −0.007, P = 0.58; DQB, FisW&C = 0.005, P = 0.80).
Pairwise comparisons did not reveal any genetic differentiation in either loci among the three sub-populations (for all pairs, F ST < 0.001; P = 0.49-0.84), suggesting intact/ongoing gene flow among them. The allelic frequencies within each sub-population and across year cohorts of the largest sub-population (N5) are shown in ESM 6.
Test of positive selection
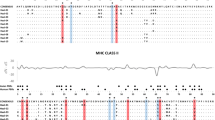
The significant deviation of the LRT statistics from a χ 2 distribution allowed rejection of the null model assuming neutral evolution (M7) in favour of a model allowing for a class of sites being subjected to diversifying selection (M8) for both loci (Table 1). In DRB, nine PSS were identified (CI 99 %, n = 7; CI 95 %, n = 2). Eight of those occurred at homologous positions with HLA-ABS, and another one was located within a three amino acid distance (Fig. 4b). In DQB, 13 PSS were detected (CI 99 %, n = 11; CI 95 %, n = 2). Six out of 13 PSS were homologous to HLA-ABS, seven other located within one to four amino acid distance (Fig. 4a). In comparison, two out of three DQB-PSS described in M. murinus (Huchard et al. 2012) were homologous to those identified in M. berthae (positions 5 and 16). Additionally, six PSS were homologous between the two species in DRB.
Amino acid variation plots for Mibe-DQB and Mibe-DRB alleles. Human antigen-binding sites (ABS) are indicated with the letter h (Bondinas et al. 2007), and positively selected sites (PSS) are indicated by black (P > 99 %) and grey triangles (P > 95 %). The insertion of two codons at positions 24–25 in seven DQB alleles causes a gap in sequences of DRB loci
The estimated values of d N and d S (±SE) through evolutionary pathways method (Table 2) confirmed the results of the first analysis by revealing an elevated d N relative to d S in all ABS versus non-ABS and in all PSS versus non-PSS. Codon-based Z-tests of selection indicated that all PSS and ABS have been affected by positive selection in both loci.
Discussion
This is a first description of the polymorphism of two MHC class II genes in the endangered Madame Berthe’s mouse lemur. We detected a total of 17 Mibe-DRB and 22 Mibe-DQB unique sequences which showed high divergence and tracks of past positive selection. Below, we compare patterns of variation and selection in M. berthae with those previously described for M. murinus, a closely related sympatric congener, that differs in several key aspects of its demography and ecology and discuss potential implications of these results on population viability.
MHC variation and selection patterns
A total of 17 Mibe-DRB and 22 Mibe-DQB unique sequences were detected within members of the three sub-populations of M. berthae in Kirindy Forest, Western Madagascar. Comparison with 59 DRB and 58 DQB sequences of M. murinus obtained previously from the same study site (Huchard et al. 2012) revealed no clear separation of amino acid sequences between the two species in either locus (Fig. 1). Nucleotide and amino acid sequence similarity between alleles of the two species, as well as the presence of a two-codon insertion located at the same position in 19 Mimu- and 7 Mibe-DQB sequences, could indicate the retention of MHC motifs in both loci during periods of time exceeding the evolutionary split between species (trans-species polymorphism; Klein 1987). MHC sequence similarity limited to exons encoding peptide binding regions have been detected many times between species that were sometimes distantly related (summarised in Klein et al. 2007; Lenz et al. 2013) and may represent examples of trans-species polymorphism or result from convergent evolution (independent evolution of similar traits in response to similar ecological pressures) (e.g. Klein et al. 2007). In addition, the reconstruction of evolutionary relationships between amino acid sequences of the two loci revealed a partial paraphyly, with three Mibe-DQB sequences clustering within DRB sequences. A BLAST search of the affected Mibe-DQB nucleotide sequences revealed Mimu-DQB sequences as a close match. Moreover, when analysed separately, Mibe sequences generated two distinct loci-specific clusters (data not shown) supporting their correct assignment to DQB loci. Additionally, these sequences occurred within multiple individuals and their presence was confirmed by replication, which excluded the possibility of sequencing run-specific amplification mismatch. Such paraphyly has been also reported previously in M. murinus by Huchard et al. (2012) and may result from a combined effect of tight physical linkage, shared origin and high functional similarity between the two loci.
In contrast to 17 Mibe-DRB and 22 Mibe-DQB sequences obtained by this study from the total of ca. 100 individuals sampled over 9 years in three study areas, 59 Mimu-DRB and 58 Mimu-DQB originated from 664 individuals of M. murinus sampled over a comparable period of time within a single study area (Huchard et al. 2012). Allelic richness (number of distinct alleles) for a given sampling effort (number of sampled individuals) that was estimated through re-sampling procedure in dataset of both species revealed the average number of distinct alleles detected for a given sampling effort to be ca. twofold lower in M. berthae compared to M. murinus (Fig. 2). This finding indicated lower allelic richness in M. berthae, where sampling of ca. 60 individuals, compared to ca. 200 individuals in M. murinus, would allow to capture most alleles present in the study population, deduced from an inflection in the graph illustrating the relationship between allelic richness and sampling effort (see Fig. 2 and Huchard et al. 2012). Moreover, the allelic distribution across year cohorts within the largest sub-population of M. berthae (N5) suggests that reported allelic richness within this study site may be overestimated, since some alleles were detected exclusively within earlier cohorts and seem to have disappeared within current generations (e.g. after 2008–2009) (ESM 6). However, sample size is too small to interpret apparent fluctuations in allelic frequencies that might be further enhanced by a progressive displacement of the M. berthae population, located at the periphery of the study area (Dammhahn and Kappeler 2008b), or by individual migrations. The lower allelic richness found in M. berthae matched our predictions based on its overall lower population densities and population size relative to M. murinus (Dammhahn and Kappeler 2005, 2008b). Even though the reasons for lower population density of M. berthae are unknown, factors such as a narrow feeding niche promoting intense intra-specific scramble competition, larger home ranges and less cohesive social networks in M. berthae (Dammhahn and Kappeler 2005, 2008a, 2009, 2010), when compared to spatially more clumped generalist M. murinus (e.g. Eberle and Kappeler 2004), are thought to contribute to the naturally lower population densities in this species (Dammhahn and Kappeler 2008b). Additionally, we observed a notable decrease in the number of newly captured individuals across the years in the most densely populated study area (N5) despite of a comparable capture effort across years (ESM 1). This pattern could either be the result of a decreasing population size or, alternatively, a spatial exclusion from the study area by its superior competitor (Dammhahn and Kappeler 2008b), whose sub-population is shifting in recent years into the areas previously exclusively occupied by M. berthae (data not shown).
Finally, the small population size, specialist diet and lower rate of social interactions among conspecifics of M. berthae could to some extent promote a limited array of pathogens and its transmission across conspecifics (reviewed in Edwards and Potts 1996; Nunn et al. 2003; Vitone et al. 2004; Rifkin et al. 2012). This could in turn result in relaxed selection, possibly manifested not only by lower allelic richness and/or divergence but also by less tracks of selection on MHC sequences. In M. berthae, allelic divergence in both loci, as well as strong evidence of past historical balancing selection on MHC sequences (Tables 1 and 2; Fig. 4), are comparable to patterns described in M. murinus (Huchard et al. 2012) and do not support the idea of a weaker pathogen-driven selection in M. berthae compared to M. murinus. Thus, population size rather than weak selection seems to constrain allelic richness in this population.
In addition, nine PSS were detected across 17 Mibe-DRB sequences and 13 across 22 Mibe-DQB sequences, suggesting that DQB may be of equal or higher functional importance than DRB in this species. This contrasts with previous findings in M. murinus, where DRB was suggested to be under stronger diversifying selection than DQB based on their relative number of PSS (11 vs. 3) (Huchard et al. 2012). Contrasting selection patterns could reflect divergent functions of this locus in M. berthae versus M. murinus. Under this scenario, we may also expect different levels of allelic variation (richness and divergence) between the two loci. This is the case in neither M. murinus nor M. berthae. This may be due to the fact that (i) selection pressures acting on both loci are not independent given their tight linkage, (ii) allelic variation reflects variation in demography and not simply selection, or (iii) allelic variation and signatures of past positive selection reflect the strength of selection over different time scales. An elevated rate of non-synonymous mutations requires a long time to accumulate (Bryja et al. 2007) as well as to vanish after the disappearance of selection (Garrigan and Hedrick 2003), whereas fluctuations in allelic variation may be more dynamic.
Implications for population resistance
To assess the adaptive significance and fitness consequences of MHC variation, it is essential to distinguish the relative importance of different measures of MHC polymorphism (Garamszegi and Nunn 2011). The high level of amino acid divergence among Mibe-alleles or their effective combination within individual genotypes may buffer potential detrimental effects of lower allelic richness for pathogen resistance. Thus, persistence of certain Mibe- alleles across several generations and study areas (ESM 6) could be facilitated by their divergence (i.e. divergent allele advantage hypothesis) (Richman et al. 2001; Schwensow et al. 2010b; Lenz et al. 2009; Lenz 2011; Froeschke and Sommer 2012; Sepil et al. 2013) or by the effect of MHC-dependent mate choice favouring specific alleles conferring resistance against dominant pathogens (e.g. Hill et al. 1991; Schad et al. 2005; Schwensow et al. 2007, 2010a; Axtner and Sommer 2012; Kloch et al. 2013). However, high allelic divergence may not be sufficient to maintain effective flexibility of the immune response in the long-term when allelic richness is low. In small populations with limited gene flow, genetic drift may weaken the capability of balancing selection to maintain high levels of MHC polymorphism through disappearance of rare allelic variants (Hartl and Clark 1997; Ejsmond and Radwan 2011). This might in turn compromise the capability of the host’s immune system to keep up with the evasive mechanisms of the current, or newly introduced pathogens. However, whether and how MHC variation found in M. berthae translates into population viability remains to be tested by the integration of genetic data with further health and survival assessment.
Overall, the empirical evidence supporting a link between MHC variation and fitness remains equivocal across taxa (reviewed in Acevedo-Whitehouse and Cunningham 2006; Radwan et al. 2010; Winternitz et al. 2013). Some populations that have undergone a demographic bottleneck seem to cope with critically low MHC variation (e.g. Ellegren et al. 1993; Mikko and Andersson 1995; Babik et al. 2005; Gangoso et al. 2012), or low MHC allelic richness compensated by high allelic divergence (e.g. Radwan et al. 2007; Castro-Prieto et al. 2011), while others retained high levels of MHC variation despite facing a bottleneck that simultaneously lowered neutral genetic diversity (Aguilar et al. 2004; Hedrick and Hurt 2012; Oliver and Piertney 2012). Although the consequences of decreasing MHC variation might be undetectable over long periods of time, it might eventually compromise the ability of small or isolated populations to resist to ever-changing pathogen pressures in the future, over time scales that may be difficult to measure in most empirical studies (reviewed in Radwan et al. 2010; Spurgin and Richardson 2010). Therefore, the continuous long-term demographic monitoring of populations for which estimates of MHC variation have been established at one or several points in time may, in the future, help us to refine our understanding of the time scale over which such processes are acting, especially in relatively short-lived species.
References
Acevedo-Whitehouse K, Cunningham AA (2006) Is MHC enough for understanding wildlife immunogenetics? Trends Ecol Evol 21:433–438. doi:10.1016/j.tree.2006.05.010
Aguilar A, Roemer G, Debenham S, Binns M, Garcelon D, Wayne RK (2004) High MHC diversity maintained by balancing selection in an otherwise genetically monomorphic mammal. Proc Natl Acad Sci U S A 101:3490–3494. doi:10.1073/pnas.0306582101
Altizer S, Nunn CL, Lindenfors P (2007) Do threatened hosts have fewer parasites? A comparative study in primates. J Anim Ecol 76:304–314. doi:10.1111/j.1365-2656.2007.01214.x
Anderson RM, May RM (1978) Regulation and stability of host–parasite population interactions. 1. Regulatory processes. J Anim Ecol 47:219–247
Andriaholinirina N, Baden A, Blanco M et al (2014) Microcebus berthae. The IUCN Red List of Threatened Species. Version 2014.3. <www.iucnredlist.org>.
Apanius V, Penn D, Slev P, Ruff LR, Potts WK (1997) The nature of selection on the Major Histocompatibility Complex. Crit Rev Immunol 17:179–224. doi:10.1615/CritRevImmunol.v17.i2.40
Averdam A, Kuschal C, Otto N et al (2011) Sequence analysis of the grey mouse lemur (Microcebus murinus) MHC class II DQ and DR region. Immunogenetics 63:85–93. doi:10.1007/s00251-010-0487-3
Axtner J, Sommer S (2012) The functional importance of sequence versus expression variability of MHC alleles in parasite resistance. Genetica 140:407–420. doi:10.1007/s10709-012-9689-y
Babik W (2010) Methods for MHC genotyping in non-model vertebrates. Mol Ecol Resour 10:237–251. doi:10.1111/j.1755-0998.2009.02788.x
Babik W, Durka W, Radwan J (2005) Sequence diversity of the MHC DRB gene in the Eurasian beaver (Castor fiber). Mol Ecol 14:4249–4257. doi:10.1111/j.1365-294X.2005.02751.x
Babik W, Taberlet P, Ejsmond MJ, Radwan J (2009) New generation sequencers as a tool for genotyping of highly polymorphic multilocus MHC system. Mol Ecol Resour 9:713–719. doi:10.1111/j.1755-0998.2009.02622.x
Baden AL, Holmes SM, Johnson SE, Engberg SE, Louis EE, Bradley BJ (2014) Species-level view of population structure and gene flow for a critically endangered primate (Varecia variegata). Ecol Evol 4:2675–2692. doi:10.1002/ece3.1119
Bernatchez L, Landry C (2003) MHC studies in nonmodel vertebrates: what have we learned about natural selection in 15 years? J Evol Biol 16:363–377. doi:10.1046/j.1420-9101.2003.00531.x
Bjorkman PJ, Burmeister WP (1994) Structures of 2 classes of MHC molecules elucidated – crucial differences and similarities. Curr Opin Struct Biol 4:852–856. doi:10.1016/0959-440X(94)90266-6
Bodmer WF (1972) Evolutionary significance of theHL-A system. Nature 237:139–183. doi:10.1038/237139a0
Bondinas GP, Moustakas AK, Papadopoulos GK (2007) The spectrum of HLA-DQ and HLA-DR alleles, 2006: a listing correlating sequence and structure with function. Immunogenetics 59:539–553. doi:10.1007/s00251-007-0224-8
Borghans JA, Beltman JB, De Boer RJ (2004) MHC polymorphism under host-pathogen coevolution. Immunogenetics 55:732–739. doi:10.1007/s00251-003-0630-5
Bryja J, Charbonnel N, Berthier K et al (2007) Density-related changes in selection pattern for Major Histocompatibility Complex genes in fluctuating populations of voles. Mol Ecol 16:5084–5097. doi:10.1111/j.1365-294X.2007.03584.x
Castro-Prieto A, Wachter B, Sommer S (2011) Cheetah paradigm revisited: MHC diversity in the world’s largest free-ranging population. Mol Biol Evol 28:1455–1468. doi:10.1093/molbev/msq330
Chikhi L, Sousa VC, Luisi P, Goossens B, Beaumont MA (2010) The confounding effects of population structure, genetic diversity and the sampling scheme on the detection and quantification of population size changes. Genetics 186:983–995. doi:10.1534/genetics.110.118661
Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890. doi:10.1093/nar/16.22.10881
Craul M, Chikhi L, Sousa V, Olivieri G, Rabesandratana A, Zimmermann E, Radespiel U (2009) Influence of forest fragmentation on an endangered large-bodied lemur in northwestern Madagascar. Biol Conserv 142:2862–2871. doi:10.1016/j.biocon.2009.05.026
Dammhahn M, Kappeler PM (2005) Social System of Microcebus berthae, the World’s Smallest Primate. Int J Primatol 26:407–435. doi:10.1007/s10764-005-2931-z
Dammhahn M, Kappeler PM (2008a) Comparative feeding ecology of sympatric Microcebus berthae and Microcebus murinus. Int J Primatol 29:1567–1589. doi:10.1007/s10764-008-9312-3
Dammhahn M, Kappeler PM (2008b) Small-scale coexistence of two mouse lemur species (Microcebus berthae and M. murinus) within a homogeneous competitive environment. Oecologia 157:473–483. doi:10.1007/s00442-008-1079-x
Dammhahn M, Kappeler PM (2009) Females go where the food is: does the socio-ecological model explain variation in social organisation of solitary foragers? Behav Ecol Sociobiol 63:939–952. doi:10.1007/s00265-009-0737-2
Dammhahn M, Kappeler PM (2010) Scramble or contest competition over food in solitarily foraging mouse lemurs (Microcebus spp.): new insights from stable isotopes. Am J Phys Anthropol 141:181–189. doi:10.1002/ajpa.21129
Dempster AP, Laird NM, Rubin DB (1977) Maximum Likelihood from Incomplete Data via the EM Algorithm. J R Stat Soc 39:1–38
Doherty PC, Zinkernagel RM (1975) Enhanced immunological surveillance in mice heterozygous at the H-2 gene complex. Nature 256:50–52. doi:10.1038/256050a0
Eberle M, Kappeler PM (2004) Sex in the dark: determinants and consequences of mixed male mating tactics in Microcebus murinus, a small solitary nocturnal primate. Behav Ecol Sociobiol 57:77–90. doi:10.1007/s00265-004-0826-1
Edwards SV, Potts WK (1996) Polymorphism of MHC genes: implications for conservation genetics of vertebrates. In: Smith TB, Wayne RK (eds) Molecular Genetic Approaches to Conservation. Oxford University Press, Oxford, pp 214–237
Eizaguirre C, Lenz TL, Traulsen A, Milinski M (2009) Speciation accelerated and stabilized by pleiotropic Major Histocompatibility Complex immunogenes. Ecol Lett 12:5–12. doi:10.1111/j.1461-0248.2008.01247.x
Eizaguirre C, Lenz TL, Kalbe M, Milinski M (2012) Divergent selection on locally adapted Major Histocompatibility Complex immune genes experimentally proven in the field. Ecol Lett 15:723–731. doi:10.1111/j.1461-0248.2012.01791.x
Ejsmond MJ, Radwan J (2011) MHC diversity in bottlenecked populations: a simulation model. Conserv Genet 12:129–137. doi:10.1007/s10592-009-9998-6
Ellegren H, Hartman G, Johansson M, Andersson L (1993) Major Histocompatibility Complex monomorphism and low levels of DNA fingerprinting variability in a reintroduced and rapidly expanding population of beavers. Proc Natl Acad Sci U S A 90:8150–8153
Excoffier L, Lischer HE (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567. doi:10.1111/j.1755-0998.2010.02847.x
Fichtel C (2012) Predation. In: Mitani JC, Call J, Kappeler P, Palombit R, Silk J (eds) The Evolution of Primate Societies. University of Chicago Press, Chicago, pp 169–194
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Froeschke G, Sommer S (2012) Insights into the complex associations between MHC class II DRB polymorphism and multiple gastrointestinal parasite infestations in the striped mouse. PLoS One 7:1–11. doi:10.1371/journal.pone.0031820
Galan M, Guivier E, Caraux G et al (2010) A 454 multiplex sequencing method for rapid and reliable genotyping of highly polymorphic genes in large-scale studies. BMC Genomics 11:296. doi:10.1186/1471-2164-11-296
Gangoso L, Alcaide M, Grande JM, Munoz J, Talbot SL, Sonsthagen SA, Sage GK, Figuerola J (2012) Colonizing the world in spite of reduced MHC variation. J Evol Biol 25:1438–1447. doi:10.1111/j.1420-9101.2012.02529.x
Ganzhorn JU, Lowry PP, Schatz GE, Sommer S (2001) The biodiversity of Madagascar: one of the world’s hottest hotspots on its way out. Oryx 35:346–348. doi:10.1046/j.1365-3008.2001.00201.x
Garamszegi LZ, Nunn CL (2011) Parasite-mediated evolution of the functional part of the MHC in primates. J Evol Biol 24:184–195. doi:10.1111/j.1420-9101.2010.02156.x
Garrigan D, Hedrick PW (2003) Perspective: detecting adaptive molecular polymorphism: lessons from the MHC. Evolution 57:1707–1722. doi:10.1554/02-732
Gilles A, Meglécz E, Pech N et al (2011) Accuracy and quality assessment of 454 GS-FLX Titanium pyrosequencing. BMC Genomics 12:245. doi:10.1186/1471-2164-12-245
Harismendy O, Ng PC, Strausberg RL et al (2009) Evaluation of next generation sequencing platforms for population targeted sequencing studies. Genome Biol 10:R32. doi:10.1186/gb-2009-10-3-r32
Hartl DL, Clark AG (1997) Principles of population genetics. Sinauer associates, Sunderland
Hedrick PW (1972) Maintenance of genetic variation with a frequency-dependent selection model as compared to the overdominant model. Genetics 72:771–775
Hedrick PW (1985) Genetics of Populations. Jones and Bartlett Publishers, Boston
Hedrick PW (1999) Balancing selection and MHC. Genetica 104:207–214. doi:10.1023/A:1026494212540
Hedrick PW (2002) Pathogen resistance and genetic variation at MHC loci. Evolution 56:1902–1908. doi:10.1111/j.0014-3820.2002.tb00116.x
Hedrick PW, Hurt CR (2012) Conservation genetics and evolution in an endangered species: research in Sonoran topminnows. Evol Appl 5:806–819. doi:10.1111/j.1752-4571.2012.00259.x
Hill AVS, Allsopp CEM, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, Bennett S, Brewster D, McMichael AJ, Greenwood BM (1991) Common West African HLA antigens are associated with protection from severe malaria. Nature 352:595–600. doi:10.1038/352595a0
Holmes SM, Baden AL, Brenneman RA, Engberg SE, Louis EE, Johnson SE (2013) Patch size and isolation influence genetic patterns in black-and-white ruffed lemur (Varecia variegata) populations. Conserv Genet 14:615–624. doi:10.1007/s10592-013-0455-1
Huchard E, Pechouskova E (2014) The major histocompatibility complex and primate behavioral ecology: new tools and future questions. Int J Primatol 35:11–31. doi:10.1007/s10764-013-9700-1
Huchard E, Albrecht C, Schliehe-Diecks S et al (2012) Large-scale MHC class II genotyping of a wild lemur population by next generation sequencing. Immunogenetics 64:895–913. doi:10.1007/s00251-012-0649-6
Huchard E, Baniel A, Schliehe-Diecks S, Kappeler PM (2013) MHC-disassortative mate choice and inbreeding avoidance in a solitary primate. Mol Ecol 22:4071–4086. doi:10.1111/mec.12349
Hughes AL, Nei M (1988) Pattern of nucleotide substitution at major histocompatibility complex class i loci reveals overdominant selection. Nature 335:167–170. doi:10.1038/335167a0
Hughes J, Page RDM (2007) Comparative tests of ectoparasite species richness in seabirds. BMC Evol Biol 7:227. doi:10.1186/1471-2148-7-227
Hughes AL, Yeager M (1998) Natural selection at major histocompatibility complex loci of vertebrates. Annu Rev Genet 32:415–434. doi:10.1146/annurev.genet.32.1.415
Kappeler (2012) The behavioral ecology of strepsirrhines and tarsiers In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB (eds) The Evolution of Primate Societies. University of Chicago Press, Chicago: 17-42.
Kappeler PM, Fichtel C (2012) Long-Term Field Studies of Primates. In: Kappeler PM, Watts DP (ed) Long term field studies of primates. Springer-Verlag Berlin Heidelberg. doi: 10.1007/978-3-642-22514-7
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241. doi:10.1016/S0169-5347(02)02489-8
Kelley J, Walter L, Trowsdale J (2005) Comparative genomics of Major Histocompatibility Complexes. Immunogenetics 56:683–695. doi:10.1007/s00251-004-0717-7
Klein J (1986) Natural history of the major histocompatibility complex. John Wiley and Sons, New York
Klein J (1987) Origin of major histocompatibility complex polymorphism: the trans-species hypothesis. Hum Immunol 19:155–162
Klein J, Sato A, Nikolaidis N (2007) MHC, TSP, and the Origin of Species: from Immunogenetics to Evolutionary Genetics. Annu Rev Genet 41:281–304. doi:10.1146/annurev.genet.41.110306.130137
Kloch A, Baran K, Buczek M, Konarzewski M, Radwan J (2013) MHC influences infection with parasites and winter survival in the root vole Microtus oeconomus. Evol Ecol 27:635–653. doi:10.1007/s10682-012-9611-1
Koboldt DC, Steinberg KM, Larson DE, Wilson RK, Mardis ER (2013) The next-generation sequencing revolution and its impact on genomics. Cell 155:27–38. doi:10.1016/j.cell.2013.09.006
Kraus C, Eberle M, Kappeler PM (2008) The costs of risky male behaviour: sex differences in seasonal survival in a small sexually monomorphic primate. Proc R Soc B 275:1635–1644. doi:10.1098/rspb. 2008.0200
Kremen C, Cameron A, Moilanen A, Phillips SJ, Thomas CD, Beentje H et al (2008) Aligning conservation priorities across taxa in Madagascar with high-resolution planning tools. Science 320:222–226. doi:10.1126/science.1155193
Lenz TL (2011) Computational prediction of MHC II-antigen binding supports divergent allele advantage and explains trans-species polymorphism. Evolution 65:2380–2390. doi:10.1111/j.1558-5646.2011.01288.x
Lenz TL, Becker S (2008) Simple approach to reduce PCR artefact formation leads to reliable genotyping of MHC and other highly polymorphic loci — implications for evolutionary analysis. Gene 427:117–123. doi:10.1016/j.gene.2008.09.013
Lenz TL, Wells K, Pfeiffer M, Sommer S (2009) Diverse MHC IIB allele repertoire increases parasite resistance and body condition in the Long-tailed giant rat (Leopoldamys sabanus). BMC Evol Biol 9:269. doi:10.1186/1471-2148-9-269
Lenz TL, Eizaguirre C, Kalbe M, Milinski M (2013) Evaluating patterns of convergent evolution and trans-species polymorphism at MHC immunogenes in two sympatric stickleback species. Evolution 67:2400–2412. doi:10.1111/evo.12124
Li M, Stoneking M (2012) A new approach for detecting low-level mutations in next-generation sequence data. Genome Biol 13:R34. doi:10.1186/gb-2012-13-5-r34
Lighten J, van Oosterhout C, Bentzen P (2014a) Critical review of NGS analyses for de novo genotyping multigene families. Mol Ecol 23:3957–3972. doi:10.1111/mec.12843
Lighten J, van Oosterhout C, Paterson IG et al (2014b) Ultra-deep Illumina sequencing accurately identifies MHC class IIb alleles and provides evidence for copy number variation in the guppy (Poecilia reticulata). Mol Ecol Resour 14:753–767. doi:10.1111/1755-0998.12225
Louis EE Jr, Ratsimbazafy JH, Razakamaharavo VR, Pierson DJ, Barber RC, Brenneman RA (2005) Conservation genetics of black-and-white ruffed lemurs, Varecia variegata, from Southeastern Madagascar. Anim Conserv 8:105–111. doi:10.1126/science.1155193
Markolf M, Roos C, Kappeler P (2008) Genetic and demographic consequences of a rapid reduction in population size in a solitary lemur (Mirza coquereli). Open Conserv Biol J 2:21–29. doi:10.2174/1874839200802010021
Mikko S, Andersson L (1995) Low major histocompatibility complex class II diversity in European and North American moose. Proc Natl Acad Sci U S A 92:4259–4263. doi:10.1073/pnas.92.10.4259
Milinski M (2006) The major histocompatibility complex, sexual selection, and mate choice. Annu Rev Ecol Evol Syst 37:159–186. doi:10.1146/annurev.ecolsys.37.091305.110242
Mittermeier RA, Konstant WR, Nicoll ME, Langrand O (1992) Lemurs of Madagascar: an action plan for their conservation, 1993–1999. IUCN, Gland, Switzerland
Nei M, Gojobori T (1986) Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol 3:418–426
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, New York
Nunn CL, Altizer S, Jones KE, Sechrest W (2003) Comparative test of parasite species richness in primates. Am Nat 162:597–614. doi:10.1086/378721
O’Brien SJ, Evermann FF (1988) Interactive influence of infectious disease and genetic diversity in natural populations. Trends Ecol Evol 3:254–259. doi:10.1016/0169-5347(88)90058-4
Oliver MK, Piertney SB (2012) Selection maintains MHC diversity through a natural population bottleneck. Mol Biol Evol 29:1713–1720. doi:10.1093/molbev/mss063
Olivieri GL, Sousa V, Chikhi L, Radespiel U (2008) From genetic diversity and structure to conservation: genetic signature of recent population declines in three mouse lemur species (Microcebus spp.). Biol Conserv 141:1257–1271. doi:10.1016/j.biocon.2008.02.025
Piertney SB, Oliver MK (2006) The evolutionary ecology of the major histocompatibility complex. Heredity (Edinb) 96:7–21. doi:10.1038/sj.hdy.6800724
Radespiel U, Rakotondravony R, Chikhi L (2008) Natural and anthropogenic determinants of genetic structure in the largest remaining population of the endangered golden-brown mouse lemur, Microcebus ravelobensis. Am J Primatol 70:860–870. doi:10.1002/ajp.20574
Radwan J, Kawalko A, Wójcik JM, Babik W (2007) MHC-DRB3 variation in a free-living population of the European bison, Bison bonasu. Mol Ecol 16:531–540. doi:10.1111/j.1365-294X.2006.03179.x
Radwan J, Biedrzycka A, Babik W (2010) Does reduced MHC diversity decrease viability of vertebrate populations? Biol Conserv 143:537–544. doi:10.1016/j.biocon.2009.07.026
Rasoloarison RM, Goodman SM, Ganzhorn U (2000) Taxonomic revision of mouse lemurs (Microcebus) in the western portions of Madagascar. Int J Primatol 21:963–1019. doi:10.1023/A:1005511129475
Razakamaharavo VR, McGuire SM, Vasey N, Louis EE, Brenneman RA (2010) Genetic architecture of two red ruffed lemur (Varecia rubra) populations of Masoala National Park. Primates 51:53–61. doi:10.1007/s10329-009-0171-0
Rensing S (1999) Immobilization and anesthesia of nonhuman primates. Primates Rep 55:33–38
Richman AD, Herrera LG, Nash D (2001) MHC class II beta sequence diversity in the deer mouse (Peromyscus maniculatus): implications for models of balancing selection. Mol Ecol 10:2765–2773. doi:10.1046/j.0962-1083.2001.01402.x
Rifkin JL, Nunn CL, Garamszegi LZ (2012) Do animals living in larger groups experience greater parasitism? A meta-analysis. Am Nat 180:70–82. doi:10.1086/666081
Rousset F, Raymond M (1995) Testing Heterozygote Excess and Defficiency. Genetics 140:1413–1419
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schad J, Sommer S, Ganzhorn JU (2004) MHC variability of a small lemur in the littoral forest fragments of southeastern madagascar. Conserv Genet 5:299–309. doi:10.1023/B:COGE.0000031137.50239.d3
Schad J, Ganzhorn JU, Sommer S (2005) Parasite burden and constitution of major histocompatibility complex in the malagasy mouse lemur, Microcebus murinus. Evolution 59:439–450. doi:10.1554/04-312
Schäffler L, Kappeler PM (2014) Distribution and abundance of the world’s smallest primate, Microcebus berthae, in Central Western Madagascar. Int J Primatol 35:557–572. doi:10.1007/s10764-014-9768-2
Schmid J, Kappeler PM (1994) Sympatric mouse lemurs (Microcebus spp.) in western Madagascar. Folia Primatol 63:162–170. doi:10.1159/000156812
Schwab D, Ganzhorn JU (2004) Distribution, population structure and habitat use of Microcebus berthae compared to those of other sympatric cheirogalids. Int J Primatol 25:307–330. doi:10.1023/B:IJOP.0000019154.17401.90
Schwensow N, Fietz J, Dausmann KH, Sommer S (2007) Neutral versus adaptive genetic variation in parasite resistance: importance of major histocompatibility complex supertypes in a free-ranging primate. Heredity (Edinb) 99:265–277. doi:10.1038/sj.hdy.6800993
Schwensow N, Eberle M, Sommer S (2008a) Compatibility counts: MHC-associated mate choice in a wild promiscuous primate. Proc Biol Sci 275:555–564. doi:10.1098/rspb.2007.1433
Schwensow N, Fietz J, Dausmann K, Sommer S (2008b) MHC-associated mating strategies and the importance of overall genetic diversity in an obligate pair-living primate. Evol Ecol 22:617–636. doi:10.1007/s10682-007-9186-4
Schwensow N, Dausmann K, Eberle M, Fietz J, Sommer S (2010a) Functional associations of similar MHC alleles and shared parasite species in two sympatric lemurs. Infect Genet Evol 10:662–668. doi:10.1016/j.meegid.2010.03.012
Schwensow N, Eberle M, Sommer S (2010b) Are there ubiquitous parasite-driven major histocompatibility complex selection mechanisms in gray mouse lemurs? Int J Primatol 31:519–537. doi:10.1007/s10764-010-9411-9
Schwitzer C, Mittermeier RA, Davies N, Johnson S, Ratsimbazafy J, Razafindramanana J, Louis EE Jr, Rajaobelina S (eds) (2013a) Lemurs of Madagascar A Strategy for Their Conservation 2013–2016. IUCN SSC Primate Specialist Group, Bristol
Schwitzer C, Mittermeier RA, Johnson SE et al (2013b) Averting lemur extinctions amid Madagascar’s political crisis. Science 343:842–843. doi:10.1126/science.1245783
Sepil I, Moghadam HK, Huchard E, Sheldon BC (2012) Characterization and 454 pyrosequencing of major histocompatibility complex class I genes in the great tit reveal complexity in a passerine system. BMC Evol Biol 12:68. doi:10.1186/1471-2148-12-68
Sepil I, Lachish S, Hinks AE, Sheldon BC (2013) Mhc supertypes confer both qualitative and quantitative resistance to avian malaria infections in a wild bird population. Proc R Soc B 280:20130134. doi:10.1098/rspb.2013.0134
Slatkin M, Excoffier L (1996) Testing for linkage disequilibrium in genotypic data using the EM algorithm. Heredity 76:377–383. doi:10.1038/hdy.1996.55
Snell GD (1968) The H-2 locus of the mouse: observations and speculations concerning its comparative genetics and its polymorphism. Folia Biol (Praha) 14:335–358
Sommer S (2005) The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front Zool 2:16. doi:10.1186/1742-9994-2-16
Sommer S, Courtiol A, Mazzoni CJ (2013) MHC genotyping of non-model organisms using next-generation sequencing: a new methodology to deal with artefacts and allelic dropout. BMC Genomics 14:542. doi:10.1186/1471-2164-14-542
Sommer S, Rakotondranary SJ, Ganzhorn JU (2014) Maintaining microendemic primate species along an environmental gradient - parasites as drivers for species differentiation. Ecol Evol 4:4751–4765. doi:10.1002/ece3.1311
Spurgin LG, Richardson DS (2010) How pathogens drive genetic diversity: MHC, mechanisms and misunderstandings. Proc Biol Sci 277:979–988. doi:10.1098/rspb.2009.2084
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
van Oosterhout C, Joyce DA, Cummings SM et al (2006) Balancing selection, random genetic drift, and genetic variation at the major histocompatibility complex in two wild populations of guppies (Poecilia reticulata). Evolution 60:2562–2574. doi:10.1111/j.0014-3820.2006.tb01890.x
Vitone ND, Altizer S, Nunn CL (2004) Body size, diet and sociality influence the species richness of parasitic worms in anthropoid primates. Evol Ecol Res 6:183–199
Weisrock DW, Rasoloarison RM, Fiorentino I, Ralison JM, Goodman SM, Kappeler PM, Yoder AD (2010) Delimiting species without nuclear monophyly in Madagascar’s mouse lemurs. PLoS One 5:e9883. doi:10.1371/journal.pone.0009883
Winternitz JC, Wares JP (2013) Duplication and population dynamics shape historic patterns of selection and genetic variation at the major histocompatibility complex in rodents. Ecol Evol 3:1552–1568. doi:10.1002/ece3.567
Winternitz JC, Minchey SG, Garamszegi LZ, Huang S, Stephens PR, Altizer S (2013) Sexual selection explains more functional variation in the mammalian major histocompatibility complex than parasitism. Proc R Soc B Biol Sci 280:20131605. doi:10.1098/rspb.2013.1605
Wright S (1965) The interpretation of population structure by F–statistics with special regard to systems of mating. Evolution 19:395–420
Wright S (1969) Evolution and the genetics of populations, vol 2: The theory of gene frequencies. University of Chicago Press, Chicago
Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24:1586–1591. doi:10.1093/molbev/msm088
Yang Z, Nielsen R, Goldman N, Pedersen AM (2000) Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 155:431–449
Yang Z, Wong WSW, Nielsen R (2005) Bayes Empirical Bayes inference of amino acid sites under positive selection. Mol Biol Evol 22:1107–1118. doi:10.1093/molbev/msi097
Zagalska-Neubauer M, Babik W, Stuglik M et al (2010) 454 sequencing reveals extreme complexity of the class II major histocompatibility complex in the collared flycatcher. BMC Evol Biol 10:395. doi:10.1186/1471-2148-10-395
Zuckerkandl E, Pauling L (1965) Evolutionary divergence and convergence in proteins. In: Evolving Genes and Proteins; Eds: Bryson V, Vogel HJ, pp. 97-166. Academic Press, New York
Acknowledgments
We would like to thank all people who have contributed to the collection and processing of DNA samples, with a special thanks to the Kirindy field assistants, notably Bruno Tsiverimana, Tiana Andrianjanahary, Jean-Claude de Beroboka and Mamy Razafindrasamba, as well as to Christina Glaschke. We also thank the Département de Biologie Animale, Université d’Antananarivo, the CAFF of the Direction des Eaux et Forêts and the CNFEREF Morondava for granting a research permit and to Prof. Lutz Walter and Dr. Christian Roos for use of the GS Junior sequencer and Nico Westphal for providing technical help. This research was funded by a SAW project of Leibniz Graduate School for the Foundations of Primate Social Behaviour issued by Wissenschaftsgemeinschaft Gottfried Wilhelm Leibniz e.V. (SAW-2011-DPZ-4), and field work was supported by the DPZ and DFG (Ka 1082/10-1&2).
Compliance with ethical standards
ᅟ
Research involving animals
We have adhered to the Guidelines for the Treatment of Animals in Behavioral Research and Teaching (Animal Behaviour 2006, 71: 245-253) and the legal requirements of the country (Madagascar) in which the fieldwork was carried out.
Funding
This study was funded by a SAW project of Leibniz Graduate School for the Foundations of Primate Social Behaviour issued by Wissenschaftsgemeinschaft Gottfried Wilhelm Leibniz e.V. (SAW-2011-DPZ-4), and field work was supported by the DPZ and DFG (Ka 1082/10-1&2).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Peter M. Kappeler and Elise Huchard contributed equally to this work.
Appendix
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Pechouskova, E., Dammhahn, M., Brameier, M. et al. MHC class II variation in a rare and ecological specialist mouse lemur reveals lower allelic richness and contrasting selection patterns compared to a generalist and widespread sympatric congener. Immunogenetics 67, 229–245 (2015). https://doi.org/10.1007/s00251-015-0827-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-015-0827-4