Abstract
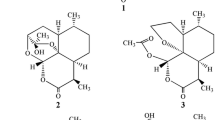
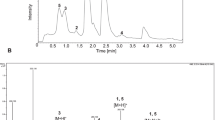
Semi-synthetic derivatives of the anti-malarial drug artemisinin hold great promise in the search for an effective and economical treatment of chloroquine-resistant forms of malaria. Unfortunately, synthetic functionalization of the artemisinin skeleton is often tedious and/or impractical. We seek to utilize 7β-hydroxyartemisinin, obtained from microbial transformation, as a semi-synthetic precursor for the synthesis of novel 7β-substituted artemisinin anti-malarial agents. Here we employ liquid cultures of Cunninghamella elegans as a means for the rational and economical bioconversion of artemisinin to 7β-hydroxyartemisinin in 78.6% yield. In addition, there were three other bioconversion products: 7β-hydroxy-9α-artemisinin (6.0%), 4α-hydroxy-1-deoxoartemisinin (5.4%), and 6β-hydroxyartemisinin (6.5%).


Similar content being viewed by others
References
Abourashed EA, Hufford CD (1996) Microbial transformation of artemether. J Nat Prod 59:251–253
Abourashed EA, Clark AM, Hufford CD (1999) Microbial models of mammalian metabolism of xenobiotics: an updated review. Curr Med Chem 6:359–374
Avery MA, Alvim-Gaston M, Woolfrey JR (1999) Synthesis and structure-activity relationships of peroxidic antimalarials based on artemisinin. Adv Med Chem 4:125–217
Azerad R (1999) Microbial models for drug metabolism. Adv Biochem Eng Biotechnol 63:169–218
Blasko G, Cordell GA (1988) Definitive 1H and 13C-NMR assignments of Artemisinin. (Qinghaosu). J Nat Prod 51:1273–1276
Ekthawatchai S, Kamchonwongpaisan S, Konsaeree P, Tarnchompoo B, Thebtaranonth Y, Yuthavong Y (2001) C-16 Artemisinin derivatives and their antimalarial and cytotoxic activities: syntheses of artemisinin monomers, dimers, trimers, and tetramers by nucleophilic additions to artemisitene. J Med Chem 44:4688–4695
Fiaux de Medeiros S, Avery MA, Avery B, Leite SGF, Freitas ACC, Williamson JS (2002) Biotransformation of 10-deoxoartemisinin to its 7β-hydroxy derivative by Mucor ramannianus. Biotechnol Lett 24:937–941
Grogan GJ, Holland HL (2000) The biocatalytic reactions of Beauveria spp. J Mol Catal B 9:1–32
Hu Y, Ziffer H, Li G, Yen HJC (1992) Microbial oxidation of the antimalarial drug arteether. Bioorg Chem 20:148–154
Hufford CD, Lee IS, El-Sohly HN, Chi HT, Baker JK (1990) Structure elucidation and thermospray high-performance liquid chromatography/mass spectroscopy (HPLC/MS) of the microbial and mammalian metabolites of the antimalarial arteether. Pharm Res 7:923–927
Hufford CD, Khalifa SI, Orabi KY, Wiggers FT (1995) 1α-Hydroxyarteether, a new microbial transformation product. J Nat Prod 58:751–755
Lee IS, Hufford CD (1990) Metabolism of antimalarial sesquiterpene lactones. Pharmacol Ther 48:345–355
Lee IS, El-Sohly HN, Croom EM, Hufford CD (1989) Microbial metabolism studies of the antimalarial sesquiterpene artemisinin. J Nat Prod 52:337–341
Mattelli A, Volonterio A, Gulletta M, Galimberti L, Maroccolo S, Gaiera G, Giani G, Rossi M, Dorigoni M, Bellina L, Orlando G, Bisoffi Z, Castelli F (2001) Malaria in illegal immigrants, Italy. Emerg Infect Dis 7:1055–1058
Orabi KY, Galay AM, Ibrahim ARS, El-Feraly FS, Khalifa SI, El-Sohly HN (1999) Microbial metabolism of artemisitene. Phytochemistry 51:257–261
Parshikov IA, Terent’ev PB, Modyanova LV (1994) Microbiological transformation in a series of nitrogen-containing heterocycles. Chem Heterocycl Compd 30:1308–1330
Posner GH, Parker MH, Northrop J, Elias JS, Ploypradith P, Xie S, Shapiro TA (1999) Orally active, hydrolytically stable, semisynthetic, antimalarial trioxanes in the artemisinin family. J Med Chem 42:300–304
Sutherland JB, Evans FE, Freeman PF, Williams AJ, Deck J, Cerniglia CE (1994) Identifications of metabolites produced by Cunninghamella elegans. Mycology 86:117–120
Zhan J, Guo H, Dai J, Zhang Y, Guo D (2002a) Microbial transformation of artemisinin by Cunninghamella echinulata and Aspergillus niger. Tetrahedron Lett 43:4519–4521
Zhan J, Zhang Y, Guo H, Han J, Ning L, Guo D (2002b) Microbial metabolism of artemisinin by Mucor polymophosporus and Aspergillus niger. J Nat Prod 65:1693–1695
Ziffer H, Hu Y, Pu Y (1992) Beauveria sulfurescens mediated oxidation of dihydroartemisinin derivatives. NATO ASI Ser C Math Phys Sci 381:361–373
Acknowledgements
We thank T.M. Heinze and J.D. McChesney for their helpful discussions and valuable input. This work was supported by Centers for Disease Control cooperative agreements U50/CCU418839 and UR3/CCU418652.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parshikov, I.A., Muraleedharan, K.M., Avery, M.A. et al. Transformation of artemisinin by Cunninghamella elegans . Appl Microbiol Biotechnol 64, 782–786 (2004). https://doi.org/10.1007/s00253-003-1524-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1524-z