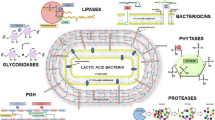
Abstract
Lactic acid bacteria (LAB) have a very long history of use in the manufacturing processes of fermented foods and a great deal of effort was made to investigate and manipulate the role of LAB in these processes. Today, the diverse group of LAB includes species that are among the best-studied microorganisms and proteolysis is one of the particular physiological traits of LAB of which detailed knowledge was obtained. The proteolytic system involved in casein utilization provides cells with essential amino acids during growth in milk and is also of industrial importance due to its contribution to the development of the organoleptic properties of fermented milk products. For the most extensively studied LAB, Lactococcus lactis, a model for casein proteolysis, transport, peptidolysis, and regulation thereof is now established. In addition to nutrient processing, cellular proteolysis plays a critical role in polypeptide quality control and in many regulatory circuits by keeping basal levels of regulatory proteins low and removing them when they are no longer needed. As part of the industrial processes, LAB are challenged by various stress conditions that are likely to affect metabolic activities, including proteolysis. While environmental stress responses of LAB have received increasing interest in recent years, our current knowledge on stress-related proteolysis in LAB is almost exclusively based on studies on L. lactis. This review provides the current status in the research of proteolytic systems of LAB with industrial relevance.


Similar content being viewed by others
References
Altermann E, Russell WM, Azcarate-Peril MA, Barrangou R, Buck BL, McAuliffe O, Souther N, Dobson A, Duong T, Callanan M, Lick S, Hamrick A, Cano R, Klaenhammer TR (2005) Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc Natl Acad Sci USA 102:3906–3912
Anastasiou R, Papadelli M, Georgalaki MD, Kalantzopoulos G, Tsakalidou E (2002) Cloning and sequencing of the gene encoding X-prolyl-dipeptidyl aminopeptidase (PepX) from Streptococcus thermophilus strain ACA-DC 4. J Appl Microbiol 93:52–59
Atlan D, Gilbert C, Blanc B, Portalier R (1994) Cloning, sequencing and characterization of the pepIP gene encoding a proline iminopeptidase from Lactobacillus delbrueckii subsp. bulgaricus CNRZ 397. Microbiology 140:527–535
Axelsson L (1998) Lactic acid bacteria: classification and physiology. In: Salminen S, von Wright A (eds) Lactic acid bacteria. Microbiology and functional aspects. Marcel Dekker, New York, pp 1–72
Azcarate-Peril MA, McAuliffe O, Altermann E, Lick S, Russell WM, Klaenhammer TR (2005) Microarray analysis of a two-component regulatory system involved in acid resistance and proteolytic activity in Lactobacillus acidophilus. Appl Environ Microbiol 71:5794–5804
Boekhorst J, Siezen RJ, Zwahlen MC, Vilanova D, Pridmore RD, Mercenier A, Kleerebezem M, de Vos WM, Brüssow H, Desiere F (2004) The complete genomes of Lactobacillusplantarum and Lactobacillus johnsonii reveal extensive differences in chromosome organization and gene content. Microbiology 150:3601–3611
Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, Ehrlich SD, Soroki A (2001) The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res 11:731–753
Bolotin A, Quinquis B, Renault P, Sorokin A, Ehrlich SD, Kulakauskas S, Lapidus A, Goltsman E, Mazur M, Pusch GD, Fonstein M, Overbeek R, Kyprides N, Purnelle B, Prozzi D, Ngui K, Masuy D, Hancy F, Burteau S, Boutry M, Delcour J, Goffeau A, Hols P (2004) Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat Biotechnol 22:1554–1558
Broadbent JR, Barnes M, Brennand C, Strickland M, Houck K, Johnson ME, Steele JL (2002) Contribution of Lactococcus lactis cell envelope proteinase specificity to peptide accumulation and bitterness in reduced-fat cheddar cheese. Appl Environ Microbiol 68:1778–1785
Buist G, Karsens H, Nauta A, van Sinderen D, Venema G, Kok J (1997) Autolysis of Lactococcus lactis caused by induced overproduction of its major autolysin, AcmA. Appl Environ Microbiol 63:2722–2728
Buist G, Venema G, Kok J (1998) Autolysis of Lactococcus lactis is influenced by proteolysis. J Bacteriol 180:5947–5953
Chaillou S, Champomier-Vergès MC, Cornet M, Crutz-Le C, Dudez AM, Martin V, Beaufils S, Darbon-Rongère E, Bossy R, Loux V, Zagorec M (2005) The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat Biotechnol 23(12):1527–1533
Champomier-Vergès MC, Marceau A, Méra T, Zagorec M (2002) The pepR gene of Lactobacillus casei is positively regulated by anaerobisis at the transcriptional level. Appl Environ Microbiol 68:3873–3877
Chapot-Chartier MP, Nardi M, Chopin MC, Chopin A, Gripon JC (1993) Cloning and sequencing of pepC, a cysteine aminopeptidase gene from Lactococcus lactis subsp. cremoris AM2. Appl Environ Microbiol 59:330–333
Chapot-Chartier MP, Rul F, Nardi M, Gripon JC (1994) Gene cloning and characterization of PepC, a cysteine aminopeptidase from Streptococcus thermophilus, with sequence similarity to the eucaryotic bleomycin hydrolase. Eur J Biochem 224:497–506
Chavagnat F, Casey MG, Meyer J (1999) Purification, characterization, gene cloning, sequencing, and overexpression of aminopeptidase N from Streptococcus thermophilus A. Appl Environ Microbiol 65:3001–3007
Chavagnat F, Meyer J, Casey MG (2000) Purification, characterisation, cloning and sequencing of the gene encoding oligopeptidase PepO from Streptococcus thermophilus A. FEMS Microbiol Lett 191:79–85
Chen YS, Steele JL (1998) Genetic characterization and physiological role of endopeptidase O from Lactobacillus helveticus CNRZ32. Appl Environ Microbiol 64:3411–3415
Chen YS, Christensen JE, Strickland M, Steele JL (2003) Identification and characterization of Lactobacillus helveticus PepO2, an endopeptidase with post-proline specificity. Appl Environ Microbiol 69:1276–1282
Christensen JE, Steele JL (2003) Impaired growth rates in milk of Lactobacillus helveticus peptidase mutants can be overcome by use of amino acid supplements. J Bacteriol 185:3297–3306
Christensen J, Lin D, Palva A, Steele J (1995) Sequence analysis, distribution and expression of an aminopeptidase gene from Lactobacillus helveticus CNRZ32. Gene 155:89–93
Christensen JE, Dudley EG, Pederson JA, Steele JL (1999) Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 76:217–246
Christensson C, Bratt H, Collins LJ, Coolbear T, Holland R, Lubbers MW, O’Toole PW, Reid JR (2002) Cloning and expression of an oligopeptidase, PepO, with novel specificity from Lactobacillus rhamnosus HN001 (DR20). Appl Environ Microbiol 68:254–262
Courtin P, Nardi M, Wegmann U, Joutsjoki V, Ogier JC, Gripon JC, Palva A, Henrich B, Monnet V (2002) Accelerating cheese proteolysis by enriching Lactococcus lactis proteolytic system with lactobacilli peptidases. Int Dairy J 12:447–454
Crow VL, Coolbear T, Gopal PK, Martley FG, McKay LL, Riepe H (1995) The role of autolysis of lactic acid bacteria in the ripening of cheese. Int Dairy J 5:855–875
den Hengst CD, van Hijum SA, Geurts JM, Nauta A, Kok J, Kuipers OP (2005a) The lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J Biol Chem 280:34332–34342
den Hengst CD, Curley P, Larsen R, Buist G, Nauta A, van Sinderen D, Kuipers OP, Kok J (2005b) Probing direct interactions between CodY and the oppD promoter of Lactococcus lactis. J Bacteriol 187:512–521
Derre I, Rapoport G, Msadek T (1999) CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol Microbiol 31:117–131
de Ruyter PG, Kuipers OP, Meijer WC, de Vos WM (1997) Food-grade controlled lysis of Lactococcus lactis for accelerated cheese ripening. Nat Biotechnol 15:976–979
Detmers FJ, Kunji ER, Lanfermeijer FC, Poolman B, Konings WN (1998) Kinetics and specificity of peptide uptake by the oligopeptide transport system of Lactococcus lactis. Biochemistry 37:16671–16679
Doeven MK, Kok J, Poolman B (2005) Specificity and selectivity determinants of peptide transport in Lactococcus lactis and other microorganisms. Mol Microbiol 57:640–649
Dudley EG, Steele JL (1994) Nucleotide sequence and distribution of the pepPN gene from Lactobacillus helveticus CNRZ32. FEMS Microbiol Lett 119:41–46
Dudley EG, Husgen AC, He W, Steele JL (1996) Sequencing, distribution, and inactivation of the dipeptidase A gene (pepDA) from Lactobacillus helveticus CNRZ32. J Bacteriol 178:701–704
Fenster KM, Parkin KL, Steele JL (1997) Characterization of a thiol-dependent endopeptidase from Lactobacillus helveticus CNRZ32. J Bacteriol 179:2529–2533
Fernández L, Bhowmik T, Steele J (1994) Characterization of the Lactobacillus helveticus CNRZ32 pepC gene. Appl Environ Microbiol 60:333–336
Fernandez-Espla MD, Rul F (1999) A new member of the aminopeptidase T family of thermophilic bacteria. Eur J Biochem 263:502–510
Fernandez-Espla MD, Garault P, Monnet V, Rul F (2000) Streptococcus thermophilus cell wall-anchored proteinase: release, purification, and biochemical and genetic characterization. Appl Environ Microbiol 66:4772–4778
Foucaud C, Kunji ER, Hagting A, Richard J, Konings WN, Desmazeaud M, Poolman B (1995) Specificity of peptide transport systems in Lactococcus lactis: evidence for a third system which transports hydrophobic di- and tripeptides. J Bacteriol 177:4652–4657
Foucaud-Scheunemann C, Poquet I (2003) HtrA is a key factor in the response to specific stress conditions in Lactococcus lactis. FEMS Microbiol Lett 224:53–59
Fox PF (1989a) The milk protein system. In: Fox P (ed) Developments in dairy chemistry, vol. 4. Elsevier Applied Science, London, pp 1–53
Fox PF (1989b) Proteolysis during cheese manufacture and ripening. J Dairy Sci 72:1379–1400
Fox PF, Wallace JM, Morgan S, Lynch CM, Niland EJ, Tobin J (1996) Acceleration of cheese ripening. Antonie Van Leeuwenhoek 70:271–297
Frees D, Ingmer H (1999) ClpP participates in the degradation of misfolded protein in Lactococcus lactis. Mol Microbiol 31:79–87
Frees D, Varmanen P, Ingmer H (2001) Inactivation of a gene that is highly conserved in gram-positive bacteria stimulates degradation of non-native proteins and concomitantly increases stress tolerance in Lactococcus lactis. Mol Microbiol 41:93–103
Gagnaire V, Piot M, Camier B, Vissers JP, Jan G, Leonil J (2004) Survey of bacterial proteins released in cheese: a proteomic approach. Int J Food Microbiol 94:185–201
Garault P, Le Bars D, Besset C, Monnet V (2002) Three oligopeptide-binding proteins are involved in the oligopeptide transport of Streptococcus thermophilus. J Biol Chem 277:32–39
Germond JE, Delley M, Gilbert C, Atlan D (2003) Determination of the domain of the Lactobacillus delbrueckii subsp. bulgaricus cell surface proteinase PrtB involved in attachment to the cell wall after heterologous expression of the prtB gene in Lactococcus lactis. Appl Environ Microbiol 69:3377–3384
Gilbert C, Atlan D, Blanc B, Portalier R (1994) Proline iminopeptidase from Lactobacillus delbrueckii subsp. bulgaricus CNRZ 397: purification and characterization. Microbiology 140:537–542
Gilbert C, Atlan D, Blanc B, Portalier R, Germond GJ, Lapierre L, Mollet B (1996) A new cell surface proteinase: sequencing and analysis of the prtB gene from Lactobacillus debrueckii subsp. bulgaricus. J Bacteriol 178:3059–3065
Gitton C, Meyrand M, Wang J, Caron C, Trubuil A, Guillot A, Mistou MY (2005) Proteomic signature of Lactococcus lactis NCDO763 cultivated in milk. Appl Environ Microbiol 71:7152–7163
Gobbetti M, Stepaniak L, De Angelis M, Corsetti A, Di Cagno R (2002) Latent bioactive peptides in milk proteins: proteolytic activation and significance in dairy processing. CRC Crit Rev Food Sci Nutr 42:223–239
Gottesman S (1996) Proteases and their targets in Escherichia coli. Annu Rev Genet 30:465–506
Guédon E, Renault P, Ehrlich D, Delorme C (2001a) Transcriptional pattern of genes coding for the proteolytic system of Lactococcus lactis and evidence for coordinated regulation of key enzymes by peptide supply. J Bacteriol 183:3614–3622
Guédon E, Serror P, Ehrlich SD, Renault P, Delorme C (2001b) Pleiotropic transcriptional repressor CodY senses the intracellular pool of branched-chain amino acids in Lactococcus lactis. Mol Microbiol 40:1227–1239
Guldtfeldt LU, Sørensen KI, Strøman P, Behrndt H, Williams D, Johansen E (2001) Effect of starter cultures with a genetically modified peptidolytic or lytic system on cheddar cheese ripening. Int Dairy J 11:373–382
Haandrikman A, Kok J, Laan H, Soemitro S, Ledeboer A, Konings W, Venema G (1989) Identification of a gene required for maturation of an extracellular lactococcal serine proteinase. J Bacteriol 171:2789–2794
Haandrikman A, Kok J, Venema G (1991) Lactococcal proteinase maturation protein PrtM is a lipoprotein. J Bacteriol 173:4517–4525
Hagting A, Kunji E, Leenhouts K, Poolman B, Konings W (1994) The di- and tripeptide transport protein of Lactococcus lactis. A new type of bacterial peptide transporter. J Biol Chem 269:11391–11399
Hebert EM, Raya RR, De Giori GS (2000) Nutritional requirements and nitrogen-dependent regulation of proteinase activity of Lactobacillus helveticus CRL 1062. Appl Environ Microbiol 66:5316–5321
Hellendoorn MA, Franke-Fayard BM, Mierau I, Venema G, Kok J (1997) Cloning and analysis of the pepV dipeptidase gene of Lactococcus lactis MG1363. J Bacteriol 179:3410–3415
Henrich B, Klein JR, Weber B, Delorme C, Renault P, Wegmann U (2002) Food-grade delivery system for controlled gene expression. Appl Environ Microbiol 68:5429–5436
Hickey RM, Ross RP, Hill C (2004) Controlled autolysis and enzyme release in a recombinant Lactococcal strain expressing the metalloendopeptidase enterolysin A. Appl Environ Microbiol 70:1744–1748
Higgins CF (1992) ABC transporters: from microorganisms to man. Annu Rev Cell Biol 67–113
Holck A, Naes H (1992) Cloning, sequencing and expression of the gene encoding the cell-envelope-associated proteinase from Lactobacillus paracasei subsp. paracasei NCDO 151. J Gen Microbiol 138:1353–1364
I’Anson K, Movahedi S, Griffin H, Gasson M, Mulholland F (1995) A non-essential glutamyl aminopeptidase is required for optimal growth of Lactococcus lactis MG1363 in milk. Microbiology 141:2873–2881
Joutsjoki V, Luoma S, Tamminen M, Kilpi M, Johansen E, Palva A (2002) Recombinant Lactococcus starters as a potential source of additional peptidolytic activity in cheese ripening. J Appl Microbiol 92:1159–1166
Juillard V, Laan H, Kunji E, Jeronimus-Stratingh CM, Bruins A, Konings W (1995) The extracellular PI-type proteinase of Lactococcus lactis hydrolyzes β-casein into more than one hundred different oligopeptides. J Bacteriol 177:3472–3478
Juillard V, Guillot A, Le Bars D, Gripon JC (1998) Specificity of milk peptide utilization by Lactococcus lactis. Appl Environ Microbiol 64:1230–1236
Katayama-Fujimura Y, Gottesman S, Maurizi MR (1987) A multiple-component, ATP-dependent protease from Escherichia coli. J Biol Chem 262:4477–4485
Klaenhammer TR, Barrangou R, Buck BL, Azcarate-Peril MA, Altermann E (2005) Genomic features of lactic acid bacteria effecting bioprocessing and health. FEMS Microbiol Rev 29:393–409
Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MW, Stiekema W, Lankhorst RM, Bron PA, Hoffer SM, Groot MN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ (2003) Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci U S A 100:1990–1995
Klein J, Klein U, Schad M, Plapp R (1993) Cloning, DNA sequence and partial characterization of pepN, a lysyl aminopeptidase from Lactobacillus delbrueckii ssp. lactis DSM 7290. Eur J Biochem 217:105–114
Klein J, Henrich B, Plapp R (1994a) Cloning and nucleotide sequence analysis of the Lactobacillus delbrueckii ssp. lactis DSM7290 cysteine aminopeptidase gene pepC. FEMS Microbiol Lett 124:291–300
Klein J, Schmidt U, Plapp R (1994b) Cloning, heterologous expression, and sequencing of a novel proline iminopeptidase gene, pepI, from Lactobacillus delbrueckii subsp. lactis DSM7290. Microbiology 140:1133–1139
Klein J, Dick A, Schick J, Matern H, Henrich B, Plapp R (1995) Molecular cloning and DNA sequence analysis of pepL, a leucyl aminopeptidase gene from Lactobacillus delbrueckii subsp. lactis DSM7290. Eur J Biochem 228:570–578
Klein JR, Schick J, Henrich B, Plapp R (1997) Lactobacillus delbrueckii subsp. lactis DSM7290 pepG gene encodes a novel cysteine aminopeptidase. Microbiology 143:527–537
Kok J, de Vos WM (1994) The proteolytic system of lactic acid bacteria. In: Gasson M, De Vos W (eds) Genetics and biotechnology of lactic acid bacteria. Blackie Academic & Professional, Glasgow, pp 169–210
Kok J, Leenhouts KJ, Haandrikman AJ, Ledeboer AM, Venema G (1988) Nucleotide sequence of the cell wall proteinase gene of Streptococcus cremoris Wg2. Appl Environ Microbiol 54:231–238
Korhonen H, Pihlanto A (2003) Food-derived bioactive peptides—opportunities for designing future foods. Curr Pharm Des 9:1297–1308
Kunji E, Hagting A, De Vries C, Juillard V, Haandrikman A, Poolman B, Konings WN (1995) Transport of β-casein-derived peptides by the oligopeptide transport system is a crucial step in the proteolytic pathway of Lactococcus lactis. J Biol Chem 270:1569–1574
Kunji ERS, Mierau I, Hagting A, Poolman B, Konings WN (1996) The proteolytic systems of lactic acid bacteria. Antonie Van Leeuwenhoek 70:187–221
Kunji ER, Fang G, Jeronimus-Stratingh CM, Bruins AP, Poolman B, Konings WN (1998) Reconstruction of the proteolytic pathway for use of beta-casein by Lactococcus lactis. Mol Microbiol 27:1107–1118
Laskowska E, Kuczynska-Wisnik D, Skorko-Glonek J, Taylor A (1996) Degradation by proteases Lon, Clp and HtrA, of Escherichia coli proteins aggregated in vivo by heat shock; HtrA protease action in vivo and in vitro. Mol Microbiol 22:555–571
Leenhouts K, Buist G, Kok J (1999) Anchoring of proteins to lactic acid bacteria. Antonie Van Leeuwenhoek 76:367–376
Leroy F, Devuyst L (2004) Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci Technol 15:67–78
Luoma S, Peltoniemi K, Joutsjoki V, Rantanen T, Tamminen M, Heikkinen I, Palva A (2001) Expression of six peptidases from Lactobacillus helveticus in Lactococcus lactis. Appl Environ Microbiol 67:1232–1238
Madkor SA, Tong PS, El Soda M (2000) Ripening of cheddar cheese with added attenuated adjunct cultures of lactobacilli. J Dairy Sci 83:1684–1691
Martínez-Cuesta MC, Fernández de Palencia P, Requena T, Peláez C (1998) Enhancement of proteolysis by a Lactococcus lactis bacteriocin producer in a cheese model system. J Agric Food Chem 46:3863–3887
Marugg JD, Meijer W, van Kranenburg R, Laverman P, Bruinenberg PG, de Vos WM (1995) Medium-dependent regulation of proteinase gene expression in Lactococcus lactis: control of transcription initiation by specific dipeptides. J Bacteriol 177:2982–2989
Matos J, Nardi M, Kumura H, Monnet V (1998) Genetic characterization of pepP, which encodes an aminopeptidase P whose deficiency does not affect Lactococcus lactis growth in milk, unlike deficiency of the X-prolyl dipeptidyl aminopeptidase. Appl Environ Microbiol 64:4591–4595
Meijer WC, Dobbelaar C, Hugenholtz J (1998) Thermoinducible lysis in Lactococcus lactis subsp. cremoris SK110: implications for cheese ripening. Int Dairy J 8:275–280
Meisel H (2004) Multifunctional peptides encrypted in milk proteins. Biofactors 21:55–61
Meisel H, Bockelman W (1999) Bioactive peptides encrypted in milk proteins: proteolytic activation and thropho-functional properties. Antonie Van Leeuwenhoek 76:207–215
Meyer J, Spahni A (1998) Influence of X-prolyl-dipeptidylaminopeptidase of Lactobacillus delbrueckii subsp. lactis on proteolysis and taste of Swiss Gruyére cheese. Milchwissenschaft 53:449–453
Meyer-Barton EC, Klein JR, Imam M, Plapp R (1993) Cloning and sequence analysis of the X-propyl-dipeptidyl-aminopeptidase gene (pepX) from Lactobacillus delbrueckii spp. lactis DSM7290. Appl Microbiol Biotechnol 40:82–89
Mierau I, Tan P, Haandrikman A, Mayo B, Kok J, Konings W, Venema G (1993) Cloning and sequencing of the gene for a lactococcal endopeptidase, an enzyme with sequence similarity to mammalian enkephalinase. J Bacteriol 175:2087–2096
Mierau I, Haandrikman A, Velterop O, Tan P, Leenhouts K, Kok J, Venema G (1994) Tripeptidase gene (pepT) of Lactococcus lactis: molecular cloning and nucleotide sequencing of pepT and construction of a chromosomal deletion mutant. J Bacteriol 176:2854–2861
Mierau I, Kunji ERS, Leenhouts KJ, Hellendoorn MA, Haandrikman AJ, Poolman B, Konings WN (1996) Multiple-peptidase mutants of Lactococcus lactis are severely impaired in their ability to grow in milk. J Bacteriol 178:2794–2803
Mierau I, Kunji ER, Venema G, Kok J (1997) Casein and peptide degradation in lactic acid bacteria. Biotechnol Genet Eng Rev 14:279–301
Monnet V, Nardi M, Chopin A, Chopin MC, Gripon JC (1994) Biochemical and genetic characterization of PepF, an oligopeptidase from Lactococcus lactis. J Biol Chem 269:32070–32076
Morel F, Frot-Coutaz J, Aubel D, Portalier R, Atlan D (1999) Characterization of a prolidase from Lactobacillus delbrueckii subsp. bulgaricus CNRZ 397 with an unusual regulation of biosynthesis. Microbiology 145:437–446
Morel F, Lamarque M, Bissardon I, Atlan D, Galinier A (2001) Autoregulation of the biosynthesis of the CcpA-like protein, PepR1, in Lactobacillus delbrueckii subsp. bulgaricus. J Mol Microbiol Biotechnol 3:63–66
Morgan S, Ross RP, Hill C (1997) Increasing starter cell lysis in cheddar cheese using a bacteriocin-producing adjunct. J Dairy Sci 80:1–10
Nakajima H, Hagting A, Kunji ER, Poolman B, Konings WN (1997) Cloning and functional expression in Escherichia coli of the gene encoding the di- and tripeptide transport protein of Lactobacillus helveticus. Appl Environ Microbiol 63:2213–2217
Nardi M, Chopin MC, Chopin A, Cals M, Gripon J (1991) Cloning and DNA sequence analysis of an X-prolyl dipeptidyl aminopeptidase gene from Lactococcus lactis subsp. lactis NCDO 763. Appl Environ Microbiol 57:45–50
Nardi M, Renault P, Monnet F (1997) Duplication of the pepF gene and shuffling of DNA fragments on the lactose plasmid of Lactococcus lactis. J Bacteriol 179:4164–4171
Navarre WW, Schneewind O (1994) Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol Microbiol 14:115–121
Nilsson D, Lauridsen AA, Tomoyasu T, Ogura T (1994) A Lactococcus lactis gene encodes a membrane protein with putative ATPase activity that is homologous to the essential Escherichia coli ftsH gene product. Microbiology 140:2601–2610
Nouaille S, Ribeiro LA, Miyoshi A, Pontes D, Le Loir Y, Oliveira SC, Langella P, Azevedo V (2003) Heterologous protein production and delivery systems for Lactococcus lactis. Genet Mol Res 2:102–111
Pastar I, Tonic I, Golic N, Kojic M, van Kranenburg R, Kleerebezem M, Topisirovic L, Jovanovic G (2003) Identification and genetic characterization of a novel proteinase, PrtR, from the human isolate Lactobacillus rhamnosus BGT10. Appl Environ Microbiol 69:5802–5811
Pederson JA, Mileski GJ, Weimer BC, Steele JL (1999) Genetic characterization of a cell envelope-associated proteinase from Lactobacillus helveticus CNRZ32. J Bacteriol 181:4592–4597
Peltoniemi K, Vesanto E, Palva A (2002) Genetic characterization of an oligopeptide transport system from Lactobacillus delbrueckii subsp. bulgaricus. Arch Microbiol 177:457–467
Petranovic D, Guedon E, Sperandio B, Delorme C, Ehrlich D, Renault P (2004) Intracellular effectors regulating the activity of the Lactococcus lactis CodY pleiotropic transcription regulator. Mol Microbiol 53:613–621
Pihlanto A, Korhonen H (2003) Bioactive peptides and proteins. Adv Food Nutr Res 47:175–276
Poolman B, Kunji E, Hagting A, Juillard V, Konings W (1995) The proteolytic pathway of Lactococcus lactis. J Appl Bacteriol 79:65–75
Poquet I, Saint V, Seznec E, Simoes N, Bolotin A, Gruss A (2000) HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol Microbiol 2000 35:1042–1051
Pridmore RD, Berger B, Desiere F, Vilanova D, Barretto C, Pittet AC, Zwahlen MC, Rouvet M, Altermann E, Barrangou R, Mollet B, Mercenier A, Klaenhammer T, Arigoni F, Schell MA (2004) The genome sequence of the probiotic intestinal bacterium Lactobacillus johnsonii NCC 533. Proc Natl Acad Sci USA 101:2512–2517
Pritchard GG, Coolbear T (1993) The physiology and biochemistry of the proteolytic system in lactic acid bacteria. FEMS Microbiol Rev 12:179–206
Rantanen T, Palva A (1997) Lactobacilli carry cryptic genes encoding peptidase-related proteins: characterization of a prolidase gene (pepQ) and a related cryptic gene (orfZ) from Lactobacillus delbrueckii subsp. bulgaricus. Microbiology 143:3899–3905
Rodríguez J, Requena T, Goudédranche H, Maubois JL, Juárez M (1996) Accelerated ripening of reduced fat semi-hard cheese from a mixture of cow’s, goat’s and ewe’s ultrafiltrated milk by using a Lac-Prt-strain of lactococci. Lait 76:513–522
Sanz Y, Toldrá F, Renault P, Poolman B (2003) Specificity of the second binding protein of the peptide ABC-transporter (Dpp) of Lactococcus lactis IL1403. FEMS Microbiol Lett 227:33–38
Savijoki K, Palva A (2000) Purification and molecular characterization of a tripeptidase (PepT) from Lactobacillus helveticus. Appl Environ Microbiol 66:794–800
Savijoki K, Ingmer H, Frees D, Vogensen FK, Palva A, Varmanen P (2003) Heat and DNA damage induction of the LexA-like regulator HdiR from Lactococcus lactis is mediated by RecA and ClpP. Mol Microbiol 50:609–621
Saxelin M, Tynkkynen S, Mattila-Sandholm T, de Vos W (2005) Probiotic and other functional microbes: from markets to mechanisms. Curr Opin Biotechnol 16:204–211
Schick J, Weber B., Klein JR, Henrich B (1999) PepR1, a CcpA-like transcription regulator of Lactobacillus delbrueckii susbp. lactis. Microbiology 145:3147–3154
Shao W, Yuksel GU, Dudley EG, Parkin KL, Steele JL (1997) Biochemical and molecular characterization of PepR, a dipeptidase, from Lactobacillus helveticus CNRZ32. Appl Environ Microbiol 63:3438–3443
Siezen RJ (1999) Multi-domain, cell-envelope proteinases of lactic acid bacteria. Antonie Van Leeuwenhoek 76:139–155
Smeds A, Varmanen P, Palva A (1998) Molecular characterization of a stress-inducible gene from Lactobacillus helveticus. J Bacteriol 180:6148–6153
Smit G, Smit BA, Engels WJM (2005) Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol Rev 29:591–610
Smukowski M, Wendorff WL, Ping Y, Rao RD (2003) Impact of cheese defects on U.S. graded cheeses. J Dairy Sci 86:364
Sridhar VR, Hughes JE, Welker DL, Broadbent JR, Steele JL (2005) Identification of endopeptidase genes from the genomic sequence of Lactobacillus helveticus CNRZ32 and the role of these genes in hydrolysis of model bitter peptides. Appl Environ Microbiol 71:3025–3032
Stefanitsi D, Sakellaris G, Garel JR (1995) The presence of two proteinases associated with the cell wall of Lactobacillus bulgaricus. FEMS Microbiol Lett 128:53–58
Strøman P (1992) Sequence of a gene (lap) encoding a 95.3-kDa aminopeptidase from Lactococcus lactis ssp. cremoris Wg2. Gene 113:107–112
Stucky K, Klein J, Schüller A, Matern H, Henrich B, Plapp R (1995) Cloning and DNA sequence analysis of pepQ, a prolidase gene from Lactobacillus delbrueckii subsp. lactis DSM7290 and partial characterization of its product. Mol Gen Genet 247:494–500
Stucky K, Schick J, Klein JR, Henrich B, Plapp R (1996) Characterization of pepR1, a gene coding for a potential transcriptional regulator of Lactobacillus delbrueckii subsp. lactis DSM7290. FEMS Microbiol Lett 136:63–69
Swaisgood H (1982) Chemistry of milk protein. In: Fox P (ed) Developments in dairy chemistry, vol. 1. Applied Science, London, pp 1–59
Tan P, van Alen-Boerrigter I, Poolman B, Siezen R, de Vos W, Konings W (1992) Characterization of the Lactococcus lactispepN encoding an aminopeptidase homologous to mammalian aminopeptidase N. FEBS Lett 306:9–16
Tuler TR, Callanan MJ, Klaenhammer TR (2002) Overexpression of peptidases in Lactococcus and evaluation of their release from leaky cells. J Dairy Sci 85:2438–2450
Tynkkynen S, Buist G, Kunji E, Kok J, Poolman B, Venema G, Haandrikman A (1993) Genetic and biochemical characterization of the oligopeptide transport system of Lactococcus lactis. J Bacteriol 175:7523–7532
Varmanen P, Vesanto E, Steele J, Palva A (1994) Characterization and expression of the pepN gene encoding a general aminopeptidase from Lactobacillus helveticus. FEMS Microbiol Lett 124:315–320
Varmanen P, Rantanen T, Palva A (1996a) An operon from Lactobacillus helveticus composed of a proline iminopeptidase gene (pepI) and two genes coding for putative members of the ABC transporter family of proteins. Microbiology 142:3459–3468
Varmanen P, Steele J, Palva A (1996b) Characterization of a prolinase gene and its product and an adjacent ABC transporter gene from Lactobacillus helveticus. Microbiology 142:809–816
Varmanen P, Rantanen T, Palva A, Tynkkynen S (1998) Cloning and characterization of a prolinase gene (pepR) from Lactobacillus rhamnosus. Appl Environ Microbiol 64:1831–1836
Varmanen P, Savijoki K, Åvall S, Palva A, Tynkkynen S (2000a) X-prolyl dipeptidyl aminopeptidase gene (pepX) is part of the glnRA operon in Lactobacillus rhamnosus. J Bacteriol 182:146–154
Varmanen P, Ingmer H, Vogensen FK (2000b) ctsR of Lactococcus lactis encodes a negative regulator of clp gene expression. Microbiology 146:1447–1455
Vermeulen N, Pavlovic M, Ehrmann MA, Gänzle MG, Vogel RF (2005) Functional characterization of the proteolytic system of Lactobacillus sanfranciscensis DSM 20451 during growth in sourdough. Appl Environ Microbiol 71:6260–6266
Vesanto E, Varmanen P, Steele J, Palva A (1994) Characterization and expression of the Lactobacillus helveticuspepC gene encoding a general aminopeptidase. Eur J Biochem 224:991–997
Vesanto E, Savijoki K, Rantanen T, Steele J, Palva A (1995) An X-prolyl dipeptidyl aminopeptidase (pepX) gene from Lactobacillus helveticus. Microbiology 141:3067–3075
Vesanto E, Peltoniemi K, Purtsi T, Steele J, Palva A (1996) Molecular characterization, over-expression and purification of a novel dipeptidase from Lactobacillus helveticus. Appl Microbiol Biotechnol 45:638–645
Vido K, Le Bars D, Mistou MY, Anglade P, Gruss A, Gaudu P (2004) Proteome analyses of heme-dependent respiration in Lactococcus lactis: involvement of the proteolytic system. J Bacteriol 186:1648–1657
Visser S, Exterkate F, Slangen C, de Veer GJCM (1986) Comparative study of action of cell wall proteinases from various strains of Streptococcus cremoris on bovine αs1-, β-, and κ-casein. Appl Microbiol Biotechnol 29:61–66
Vongerichten KF, Klein JR, Matern H, Plapp R (1994) Cloning and nucleotide sequence analysis of pepV, a carnosinase gene from Lactobacillus delbrueckii subsp. lactis DSM7290, and partial characterization of the enzyme. Microbiology 140:2591–2600
Wegmann U, Klein R, Drumm I, Kuipers OP, Henrich B (1999) Introduction of peptidase genes from Lactobacillus delbrueckii subsp. lactis into Lactococcus lactis and controlled expression. Appl Environ Microbiol 65:4729–4733
Yüksel GU, Steele JL (1996) DNA sequence analysis, expression, distribution, and physiological role of the Xaa-prolyl dipeptidyl aminopeptidase gene from Lactobacillus helveticus CNRZ32. Appl Microbiol Biotechnol 44:766–773
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Savijoki, K., Ingmer, H. & Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl Microbiol Biotechnol 71, 394–406 (2006). https://doi.org/10.1007/s00253-006-0427-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0427-1