Abstract
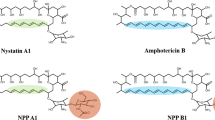
Polyene antibiotics such as nystatin are a large family of very valuable antifungal polyketide compounds typically produced by soil actinomycetes. Previously, using a polyene cytochrome P450 hydroxylase-specific genome screening strategy, Pseudonocardia autotrophica KCTC9441 was determined to contain an approximately 125.7-kb region of contiguous DNA with a total of 23 open reading frames, which are involved in the biosynthesis and regulation of a structurally unique polyene natural product named NPP. Here, we report the complete structure of NPP, which contains an aglycone identical to nystatin and harbors a unique di-sugar moiety, mycosaminyl-(α1-4)-N-acetyl-glucosamine. A mutant generated by inactivation of a sole glycosyltransferase gene (nppDI) within the npp gene cluster can be complemented in trans either by nppDI-encoded protein or by its nystatin counterpart, NysDI, suggesting that the two sugars might be attached by two different glycosyltransferases. Compared with nystatin (which bears a single sugar moiety), the di-sugar containing NPP exhibits approximately 300-fold higher water solubility and 10-fold reduced hemolytic activity, while retaining about 50% antifungal activity against Candida albicans. These characteristics reveal NPP as a promising candidate for further development into a pharmacokinetically improved, less-cytotoxic polyene antifungal antibiotic.





Similar content being viewed by others
References
Al-Hamidi H, Edwards AA, Mohammad MA, Nokhodchi A (2010) To enhance dissolution rate of poorly water-soluble drugs: glucosamine hydrochloride as a potential carrier in solid dispersion formulations. Colloid Surf B Bioint 76:170–178
Aparicio JF, Colina AJ, Ceballos E, Martín JF (1999) The biosynthetic gene cluster for the 26-membered ring polyene macrolide pimaricin: a new polyketide synthase organization encoded by two subclusters separated by functionalization genes. J Biol Chem 274:10133–10139
Aparicio JF, Fouces R, Mendes MV, Olivera N, Martín JF (2000) A complex multienzyme system encoded by five polyketide synthase genes is involved in the biosynthesis of the 26-membered polyene macrolide pimaricin in Streptomyces natalensis. Chem Biol 7:895–905
Aparicio JF, Caffrey P, Gil JA, Zotchev SB (2003) Polyene antibiotic biosynthesis gene clusters. Appl Microbiol Biotechnol 61:179–188
Bierman M, Logan R, O’Brien K, Seno ET, Nagaraja Rao R, Schoner BE (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49
Bolard J (1986) How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta Rev Biomembr 864:257–304
Borgos SEF, Tsan P, Sletta H, Ellingsen TE, Lancelin J-M, Zotchev SB (2006) Probing the structure-function relationship of polyene macrolides: engineered biosynthesis of soluble nystatin analogues. J Med Chem 49:2431–2439
Brautaset T, Sekurova ON, Sletta H, Ellingsen TE, Strøm AR, Valla S, Zotchev SB (2000) Biosynthesis of the polyene antifungal antibiotic nystatin in Streptomyces noursei ATCC 11455: analysis of the gene cluster and deduction of the biosynthetic pathway. Chem Biol 7:395–403
Brautaset T, Bruheim P, Sletta H, Hagen L, Ellingsen TE, Strøm AR, Valla S, Zotchev SB (2002) Hexaene derivatives of nystatin produced as a result of an induced rearrangement within the nysC polyketide synthase gene in S. noursei ATCC 11455. Chem Biol 9:367–373
Brautaset T, Sletta H, Nedal A, Borgos SEF, Degnes KF, Bakke I, Volokhan O, Sekurova ON, Treshalin ID, Mirchink EP, Dikiy A, Ellingsen TE, Zotchev SB (2008) Improved antifungal polyene macrolides via engineering of the nystatin biosynthetic genes in Streptomyces noursei. Chem Biol 15:1198–1206
Bruheim P, Borgos SEF, Tsan P, Sletta H, Ellingsen TE, Lancelin J-M, Zotchev SB (2004) Chemical diversity of polyene macrolides produced by Streptomyces noursei ATCC 11455 and recombinant strain ERD44 with genetically altered polyketide synthase NysC. Antimicrob Agents Chemother 48:4120–4129
Byrne B, Carmody M, Gibson E, Rawlings B, Caffrey P (2003) Biosynthesis of deoxyamphotericins and deoxyamphoteronolides by engineered strains of Streptomyces nodosus. Chem Biol 10:1215–1224
Caffrey P, Lynch S, Flood E, Finnan S, Oliynyk M (2001) Amphotericin biosynthesis in Streptomyces nodosus: deductions from analysis of polyketide synthase and late genes. Chem Biol 8:713–723
Campelo AB, Gil JA (2002) The candicidin gene cluster from Streptomyces griseus IMRU 3570. Microbiology 148:51–59
Carmody M, Murphy B, Byrne B, Power P, Rai D, Rawlings B, Caffrey P (2005) Biosynthesis of amphotericin derivatives lacking exocyclic carboxyl groups. J Biol Chem 280:34420–34426
Coutinho A, Silva L, Fedorov A, Prieto M (2004) Cholesterol and ergosterol influence nystatin surface aggregation: relation to pore formation. Biophys J 87:3264–3276
Enoch DA, Ludlam HA, Brown NM (2006) Invasive fungal infections: a review of epidemiology and management options. J Med Microbiol 55:809–818
Falk R, Domb AJ, Polacheck I (1999) A novel injectable water-soluble amphotericin B-arabinogalactan conjugate. Antimicrob Agents Chemother 43:1975–1981
Gupte M, Kulkarni P, Ganguli BN (2002) Antifungal antibiotics. Appl Microbiol Biotechnol 58:46–57
Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schrempf H (eds) (1985) Genetic manipulation of Streptomyces: A laboratory manual. John Innes Foundation, Norwich
Hu Y, Walker S (2002) Remarkable structural similarities between diverse glycosyltransferases. Chem Biol 9:1287–1296
Jeon H-G, Seo J, Lee M-J, Han K, Kim E-S (2011) Analysis and functional expression of NPP pathway-specific regulatory genes in Pseudonocardia autotrophica. J Ind Microbiol Biotechnol 38:573–579
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (eds) (2000) Practical Streptomyces genetics. The John Innes Foundation, Norwich
Kim B-G, Lee M-J, Seo J, Hwang Y-B, Lee M-Y, Han K, Sherman DH, Kim E-S (2009) Identification of functionally clustered nystatin-like biosynthetic genes in a rare actinomycetes, Pseudonocardia autotrophica. J Ind Microbiol Biotechnol 36:1425–1434
Kurita K, Matsumura Y, Takahara H, Hatta K, Shimojoh M (2011) Synthesis and macrophage activation of lentinan-mimic branched amino polysaccharides: curdlans having N-acetyl-d-glucosamine branches. Biomacromolecules 12:2267–2274
MacNeil DJ, Gewain KM, Ruby CL, Dezeny G, Gibbons PH, MacNeil T (1992) Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 111:61–68
Nedal A, Sletta H, Brautaset T, Borgos SEF, Sekurova ON, Ellingsen TE, Zotchev SB (2007) Analysis of the mycosamine biosynthesis and attachment genes in the nystatin biosynthetic gene cluster of Streptomyces noursei ATCC 11455. Appl Environ Microbiol 73:7400–7407
Nosanchuk JD (2006) Current status and future of antifungal therapy for systemic mycoses. Recent patents on anti-infective drug discovery 1:75-84
Sambrook J, Fritsch EF, Maniatis T (eds) (1989) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Seco EM, Cuesta T, Fotso S, Laatsch H, Malpartida F (2005) Two polyene amides produced by genetically modified Streptomyces diastaticus var. 108. Chem Biol 12:535–543
Silveira FP, Husain S (2007) Fungal infections in solid organ transplantation. Med Mycol 45:305–320
Sims CR, Ostrosky-Zeichner L, Rex JH (2005) Invasive candidiasis in immunocompromised hospitalized patients. Arch Med Res 36:660–671
Sletta H, Borgos SEF, Bruheim P, Sekurova ON, Grasdalen H, Aune R, Ellingsen TE, Zotchev SB (2005) Nystatin biosynthesis and transport: nysH and nysG genes encoding a putative ABC transporter system in Streptomyces noursei ATCC 11455 are required for efficient conversion of 10-deoxynystatin to nystatin. Antimicrob Agents Chemother 49:4576–4583
Sowinski P, Pawlak JK, Borowski E, Iwashita T (1985) The structure of amphotericin A. II. The complete structure of the antibiotic. J Antibiot 38:175–180
Thibodeaux CJ, Liu HW (2007) Manipulating nature’s sugar biosynthetic machineries for glycodiversification of macrolides: recent advances and future prospects. Pure Appl Chem 79:785–799
Treshchalin ID, Sletta H, Borgos SEF, Pereverzeva ER, Voeikova TA, Ellingsen TE, Zotchev SB (2005) Comparative analysis of in vitro antifungal activity and in vivo acute toxicity of the nystatin analogue S44HP produced via genetic manipulation. Antibiot Khimioter 50:18–22
Volokhan O, Sletta H, Ellingsen TE, Zotchev SB (2006) Characterization of the P450 monooxygenase NysL, responsible for C-10 hydroxylation during biosynthesis of the polyene macrolide antibiotic nystatin in Streptomyces noursei. Appl Environ Microbiol 72:2514–2519
Volpon L, Lancelin J-M (2002) Solution NMR structure of five representative glycosylated polyene macrolide antibiotics with a sterol-dependent antifungal activity. Eur J Biochem 269:4533–4541
Walsh CT, Losey HC, Freel Meyers CL (2003) Antibiotic glycosyltransferases. Biochem Soc Trans 31:487–492
Wang J, Li J, Chen H-N, Chang H, Tanifum CT, Liu H-H, Czyryca PG, Chang C-T (2005) Glycodiversification for the optimization of the kanamycin class aminoglycosides. J Med Chem 48:6271–6285
Zotchev SB (2003) Polyne macrolide antibiotics and their applications in human therapy. Curr Med Chem 10:211–223
Zotchev S, Haugan K, Sekurova O, Sletta H, Ellingsen TE, Valla S (2000) Identification of a gene cluster for antibacterial polyketide-derived antibiotic biosynthesis in the nystatin producer Streptomyces noursei ATCC 11455. Microbiology 146:611–619
Acknowledgements
This work was supported by the 21C Frontier Microbial Genomics and Applications Center Program, Ministry of Education, Science and Technology, Republic of Korea and the National Research Foundation of Korea (NRF) Grant (NRF-2010-616-D00030). This work was also supported in part by the grants from the 973 programs of Ministry of Science and Technology and the National Natural Science Foundation of China (D.K., L.B., Z.D., and S.L.), as well as NIH grant R01 GM076477 and the Hans W. Vahlteich Professorship (D.H.S.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Mi-Jin Lee and Dekun Kong equally contributed to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 466 kb)
Rights and permissions
About this article
Cite this article
Lee, MJ., Kong, D., Han, K. et al. Structural analysis and biosynthetic engineering of a solubility-improved and less-hemolytic nystatin-like polyene in Pseudonocardia autotrophica . Appl Microbiol Biotechnol 95, 157–168 (2012). https://doi.org/10.1007/s00253-012-3955-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-3955-x