Abstract
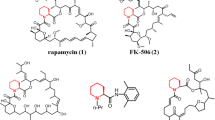
l-Pipecolic acid is a key component of biologically active molecules and a pharmaceutically important chiral building block. It can be stereoselectively produced from l-lysine by a two-step bioconversion involving l-lysine α-oxidase and ∆1-piperideine-2-carboxylae (Pip2C) reductase. In this study, we focused on an l-lysine α-oxidase from Scomber japonicus that was originally identified as an apoptosis-inducing protein (AIP) and applied the enzyme to one-pot fermentation of l-pipecolic acid in Escherichia coli. A synthetic gene coding for an AIP was expressed in E. coli, and the recombinant enzyme was purified and characterized. The purified enzyme was determined to be a homodimer with a molecular mass of 133.9 kDa. The enzyme essentially exhibited the same substrate specificity as the native enzyme. Optimal temperature and pH for the enzymatic reaction were 70 °C and 7.4, respectively. The enzyme was stable below 60 °C and at a pH range of 5.5–7.5 but was markedly inhibited by Co2+. To establish a one-pot fermentation system for the synthesis of optically pure l-pipecolic acid from dl-lysine, an E. coli strain carrying a plasmid encoding AIP, Pip2C reductase from Pseudomonas putida, lysine racemase from P. putida, and glucose dehydrogenase from Bacillus subtilis was constructed. The one-pot process produced 45.1 g/L of l-pipecolic acid (87.4 % yield from dl-lysine) after a 46-h reaction with high optical purity (>99.9 % enantiomeric excess).





Similar content being viewed by others
References
Aketa K, Terashima S, Yamada S (1976) Stereochemical studies XL. A biomimetic conversion of l-lysine into optically active 2-substituted piperidines. Syntheses of d- and l-pipecolic acid, and (S)(+)-coniine from l-lysine. Chem Pharm Bull 24:621–631
Asada K, Katou R, Kawabata H, Miyake R (2012) Novel hydrolase protein. WO-patent 2012029819A1 (to AIP Corporation)
Atanas DR, Luke AM (2013) Amino acid racemization in Pseudomonas putida KT2440. J Bacteriol 195:5016–5024. doi:10.1128/JB.00761-13
Boger DL, Chen JH, Saionz KW (1996) (-)-Sandramycin: total synthesis and characterization of DNA binding properties. J Am Chem Soc 118:1629–1644. doi:10.1021/Ja952799y
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1006/Abio.1976.9999
Calmes M, Escale FO, Rolland M, Martinez J (2003) Diastereoselective esterification of (+/-)-N-trifluoroacetyl pipecolic acid using (S)-α-methyl pantolactone: synthesis of (S)-N-Boc-pipecolic acid and (S)-N-Boc-2-piperidinemethanol. Tetrahedron Asymmetry 14:1685–1689. doi:10.1016/S0957-4166(03)00304-5
Chang MY, Kung YH, Wu TC (2006) Synthesis of pipecolic acid and baikiain. Heterocycles 68:2365–2373
Chattopadhyay SK, Biswas T, Biswas T (2008) Complementary routes to both enantiomers of pipecolic acid and 4,5-dihydroxypipecolic acid derivatives. Tetrahedron Lett 49:1365–1369. doi:10.1016/J.Tetlet.2007.12.097
Clevenstine EC, Walter P, Harris TM, Broquist HP (1979) Biosynthesis of slaframine, (1S,6S,8aS)-1-acetoxy-6-aminooctahydroindolizine, parasympathomimetic alkaloid of fungal origin. 4. Metabolic fate of ethyl pipecolylacetate, 1,3-dioxooctahydroindolizine, and 1-hydroxyoctahydroindolizine in Rhizoctonia leguminicola. Biochemistry 18:3663–3667. doi:10.1021/Bi00584a004
Eichhorn E, Roduit JP, Shaw N, Heinzmann K, Kiener A (1997) Preparation of (S)-piperazine-2-carboxylic acid, (R)-piperazine-2-carboxylic acid, and (S)-piperidine-2-carboxylic acid by kinetic resolution of the corresponding racemic carboxamides with stereoselective amidases in whole bacterial cells. Tetrahedron Asymmetry 8:2533–2536. doi:10.1016/S0957-4166(97)00256-5
Ekenstam TBA, Bovin C (1987) L-N-n-propylpipecolic acid-2,6-xylidide. US patent US4695576A
Flynn GA, Giroux EL, Dage RC (1987) An acyl-iminium ion cyclization route to a novel conformationally restricted dipeptide mimic—applications to angiotensin-converting enzyme-inhibition. J Am Chem Soc 109:7914–7915. doi:10.1021/Ja00259a068
Fujii T, Aritoku Y, Agematu H, Tsunekawa H (2002a) Increase in the rate of l-pipecolic acid production using lat-expressing Escherichia coli by lysP and yeiE amplification. Biosci Biotechnol Biochem 66:1981–1984. doi:10.1271/bbb.66.1981
Fujii T, Mukaihara M, Agematu H, Tsunekawa H (2002b) Biotransformation of l-lysine to l-pipecolic acid catalyzed by l-lysine 6-aminotransferase and pyrroline-5-carboxylate reductase. Biosci Biotechnol Biochem 66:622–627. doi:10.1271/bbb.66.622
Fujita Y, Ramaley R, Freese E (1977) Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus sublitis. J Bacteriol 132:282–293
Gatto GJ, Boyne MT, Kelleher NL, Walsh CT (2006) Biosynthesis of pipecolic acid by RapL, a lysine cyclodeaminase encoded in the rapamycin gene cluster. J Am Chem Soc 128:3838–3847. doi:10.1021/Ja0587603
Germann UA, Shlyakhter D, Mason VS, Zelle RE, Duffy JP, Galullo V, Armistead DM, Saunders JO, Boger J, Harding MW (1997) Cellular and biochemical characterization of VX-710 as a chemosensitizer: reversal of P-glycoprotein-mediated multidrug resistance in vitro. Anti Cancer Drug 8:125–140. doi:10.1097/00001813-199702000-00004
Ginesta X, Pericas MA, Riera A (2002) Straightforward entry to the pipecolic acid nucleus. Enantioselective synthesis of baikiain. Tetrahedron Lett 43:779–782. doi:10.1016/S0040-4039(01)02271-7
Goto M, Muramatsu H, Mihara H, Kurihara T, Esaki N, Omi R, Miyahara I, Hirotsu K (2005) Crystal structures of ∆1-piperideine-2-carboxylate/∆1-pyrroline-2-carboxylate reductase belonging to a new family of NAD(P)H-dependent oxidoresuctases—conformational change, substrate recognition, and stereochemistry of the reaction. J Biol Chem 280:40875–40884. doi:10.1074/Jbc.M507399200
Hamilton GS, Wu YQ, Limburg DC, Wilkinson DE, Vaal MJ, Li JH, Thomas C, Huang W, Sauer H, Ross DT, Soni R, Chen Y, Guo HS, Howorth P, Valentine H, Liang S, Spicer D, Fuller M, Steiner JP (2002) Synthesis of N-glyoxyl prolyl and pipecolyl amides and thioesters and evaluation of their in vitro and in vivo nerve regenerative effects. J Med Chem 45:3549–3557. doi:10.1021/Jm010556c
Job V, Marcone GL, Pilone MS, Pollegioni L (2002) Glycine oxidase from Bacillus subtilis. Characterization of a new flavoprotein. J Biol Chem 277:6985–6993. doi:10.1074/jbc.M111095200
Jung SK, Mai A, Iwamoto M, Arizono N, Fujimoto D, Sakamaki K, Yonehara S (2000) Purification and cloning of an apoptosis-inducing protein derived from fish infected with Anisakis simplex, a causative nematode of human anisakiasis. J Immunol 165:1491–1497. doi:10.4049/jimmunol.165.3.1491
Kasai K, Ishikawa T, Komata T, Fukuchi K, Chiba M, Nozaka H, Nakamura T, Sato T, Miura T (2010) Novel l-amino acid oxidase with antibacterial activity against methicillin-resistant Staphylococcus aureus isolated from epidermal mucus of the flounder Platichthys stellatus. FEBS J 277:453–465. doi:10.1111/j.1742-4658.2009.07497.x
Khaw LE, Bohm GA, Metcalfe S, Staunton J, Leadlay PF (1998) Mutational biosynthesis of novel rapamycins by a strain of Streptomyces hygroscopicus NRRL 5491 disrupted in rapL, encoding a putative lysine cyclodeaminase. J Bacteriol 180:809–814
Kikumoto R, Tamao Y, Tezuka T, Tonomura S, Hara H, Ninomiya K, Hijikata A, Okamoto S (1984) Selective inhibition of thrombin by (2R,4R)-4-methyl-1-[N 2-[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl)sulfonyl]-l-arginyl)]-2-piperidinecarboxylic acid. Biochemistry 23:85–90
Kitani Y, Mori T, Nagai H, Toyooka K, Ishizaki S, Shimakura K, Shiomi K, Nagashima Y (2007a) Gene expression and distribution of antibacterial l-amino acid oxidase in the rockfish Sebastes schlegeli. Fish Shellfish Immunol 23:1178–1186. doi:10.1016/j.fsi.2007.04.005
Kitani Y, Tsukamoto C, Zhang G, Nagai H, Ishida M, Ishizaki S, Shimakura K, Shiomi K, Nagashima Y (2007b) Identification of an antibacterial protein as l-amino acid oxidase in the skin mucus of rockfish Sebastes schlegeli. FEBS J 274:125–136. doi:10.1111/j.1742-4658.2006.05570.x
Kitani Y, Toyooka K, Endo M, Ishizaki S, Nagashima Y (2013) Intra-tissue localization of an antibacterial l-amino acid oxidase in the rockfish Sebastes schlegeli. Dev Comp Immunol 39:456–459. doi:10.1016/j.dci.2012.12.008
Kusakabe H, Kodama K, Kuninaka A, Yoshino H, Misono H, Soda K (1980) A new antitumor enzyme, l-lysine α-oxidase from Trichoderma viride. Purification and enzymological properties. J Biol Chem 255:976–981
Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227:680–685. doi:10.1038/227680a0
Lampel KA, Uratani B, Chaudhry GR, Ramaley RF, Rudikoff S (1986) Characterization of the developmentally regulated Bacillus subtilis glucose dehydrogenase gene. J Bacteriol 166:238–243
Macheroux P (1999) UV-visible spectroscopy as a tool to study flavoproteins. Methods Mol Biol 131:1–7. doi:10.1385/1-59259-266-X:1
Martin J, Plaquevent JC, Maddaluno J, Rouden J, Lasne MC (2009) Efficient deracemization of pipecolic acid amides through enantioselective protonation of their lithium enolates: insights into the origin of the transferred proton. Eur J Org Chem 131:5414–5422. doi:10.1002/Ejoc.200900726
Mihara H, Muramatsu H, Kakutani R, Yasuda M, Ueda M, Kurihara T, Esaki N (2005) N-Methyl-l-amino acid dehydrogenase from Pseudomonas putida. A novel member of an unusual NAD(P)-dependent oxidoreductase superfamily. FEBS J 272:1117–1123. doi:10.1111/j.1742-4658.2004.04541.x
Morimoto Y, Honda K, Ye X, Okano K, Ohtake H (2014) Directed evolution of thermotolerant malic enzyme for improved malate production. J Biosci Bioeng 117:147–152. doi:10.1016/j.jbiosc.2013.07.005
Murakawa M, Jung SK, Iijima K, Yonehara S (2001) Apoptosis-inducing protein, AIP, from parasite-infected fish induces apoptosis in mammalian cells by two different molecular mechanisms. Cell Death Differ 8:298–307. doi:10.1038/sj.cdd.4400811
Muramatsu H, Mihara H, Kakutani R, Yasuda M, Ueda M, Kurihara T, Esaki N (2005) The putative malate/lactate dehydrogenase from Pseudomonas putida is an NADPH-dependent ∆1-piperideine-2-carboxylate/∆1-pyrroline-2-carboxylate reductase involved in the catabolism of d-lysine and d-proline. J Biol Chem 280:5329–5335. doi:10.1074/jbc.M411918200
Muramatsu H, Mihara H, Yasuda M, Ueda M, Kurihara T, Esaki N (2006) Enzymatic synthesis of l-pipecolic acid by ∆1-piperideine-2-carboxylate reductase from Pseudomonas putida. Biosci Biotechnol Biochem 70:2296–2298. doi:10.1271/Bbb.60125
Nagashima Y, Tsukamoto C, Kitani Y, Ishizaki S, Nagai H, Yanagimoto T (2009) Isolation and cDNA cloning of an antibacterial l-amino acid oxidase from the skin mucus of the great sculpin Myoxocephalus polyacanthocephalus. Comp Biochem Physiol B: Biochem Mol Biol 154:55–61. doi:10.1016/j.cbpb.2009.05.006
Nakatsuka M, Ragan JA, Sammakia T, Smith DB, Uehling DE, Schreiber SL (1990) Total synthesis of FK506 and an FKBP probe reagent, (C8, C9–13C2)-FK506. J Am Chem Soc 112:5583–5601. doi:10.1021/Ja00170a024
Picaud S, Olsson ME, Brodelius PE (2007) Improved conditions for production of recombinant plant sesquiterpene synthases in Escherichia coli. Protein Expr Purif 51:71–79. doi:10.1016/j.pep.2006.06.025
Pollegioni L, Motta P, Molla G (2013) l-Amino acid oxidase as biocatalyst: a dream too far? Appl Microbiol Biotechnol 97:9323–9341. doi:10.1007/S00253-013-5230-1
Ramaley RF, Vasantha N (1983) Glycerol protection and purification of Bacillus subtilis glucose dehydrogenase. J Biol Chem 258:12558–12565
Rogers LMA, Rouden J, Lecomte L, Lasne MC (2003) Enantioselective decarboxylation-reprotonation of an α-amino malonate derivative as a route to optically enriched cyclic α-amino acid. Tetrahedron Lett 44:3047–3050. doi:10.1016/S0040-4039(03)00557-4
Sandee D, Tungpradabkul S, Kurokawa Y, Fukui K, Takagi M (2005) Combination of Dsb coexpression and an addition of sorbitol markedly enhanced soluble expression of single-chain Fv in Escherichia coli. Biotechnol Bioeng 91:418–424. doi:10.1002/bit.20524
Takayama S, Isogai A, Nakata M, Suzuki H, Suzuki A (1984) Structure of Cyl-1, a novel cyclotetrapeptide from Cylindrocladium scoparium. Agric Biol Chem Tokyo 48:839–842
Tanaka H, Kuroda A, Marusawa H, Hatanaka H, Kino T, Goto T, Hashimoto M, Taga T (1987) Structure of FK506 -a novel Immunosuppressant isolated from Streptomyces. J Am Chem Soc 109:5031–5033. doi:10.1021/Ja00250a050
Watanabe LA, Haranaka S, Jose B, Yoshida M, Kato T, Moriguchi M, Soda K, Nishino N (2005) An efficient access to both enantiomers of pipecolic acid. Tetrahedron Asymmetry 16:903–908. doi:10.1016/J.Tetasy.2005.01.017
Weigelt S, Huber T, Hofmann F, Jost M, Ritzefeld M, Luy B, Freudenberger C, Majer Z, Vass E, Greie JC, Panella L, Kaptein B, Broxterman QB, Kessler H, Altendorf K, Hollosi M, Sewald N (2012) Synthesis and conformational analysis of efrapeptins. Chemistry 18:478–487. doi:10.1002/Chem.201102134
Yasuda M, Ueda M, Muramatsu H, Mihara H, Esaki N (2006) Enzymatic synthesis of cyclic amino acids by N-methyl-l-amino acid dehydrogenase from Pseudomonas putida. Tetrahedron Asymmetry 17:1775–1779. doi:10.1016/J.Tetasy.2006.07.005
Acknowledgments
This work was supported in part by a research grant (to H. M.) from the Ritsumeikan Global Innovation Research Organization (R-GIRO), Ritsumeikan University.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tani, Y., Miyake, R., Yukami, R. et al. Functional expression of l-lysine α-oxidase from Scomber japonicus in Escherichia coli for one-pot synthesis of l-pipecolic acid from dl-lysine. Appl Microbiol Biotechnol 99, 5045–5054 (2015). https://doi.org/10.1007/s00253-014-6308-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6308-0