Abstract
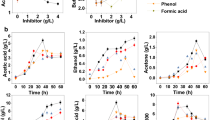
In addition to glucans, xylans, and arabinans, lignocellulosic biomass hydrolysates contain significant levels of nonsugar components that are toxic to the microbes that are typically used to convert biomass to biofuels and chemicals. To enhance the tolerance of acetone–butanol–ethanol (ABE)-generating Clostridium beijerinckii NCIMB 8052 to these lignocellulose-derived microbial inhibitory compounds (LDMICs; e.g., furfural), we have been examining different metabolic perturbation strategies to increase the cellular reductant pools and thereby facilitate detoxification of LDMICs. As part of these efforts, we evaluated the effect of allopurinol, an inhibitor of NAD(P)H-generating xanthine dehydrogenase (XDH), on C. beijerinckii grown in furfural-supplemented medium and found that it unexpectedly increased the rate of detoxification of furfural by 1.4-fold and promoted growth, butanol, and ABE production by 1.2-, 2.5-, and 2-fold, respectively. Since NAD(P)H/NAD(P)+ levels in C. beijerinckii were largely unchanged upon allopurinol treatment, we postulated and validated a possible basis in DNA repair to account for the solventogenic gains with allopurinol. Following the observation that supplementation of allopurinol in the C. beijerinckii growth media mitigates the toxic effects of nalidixic acid, a DNA-damaging antibiotic, we found that allopurinol elicited 2.4- and 6.7-fold increase in the messenger RNA (mRNA) levels of xanthine and hypoxanthine phosphoribosyltransferases, key purine-salvage enzymes. Consistent with this finding, addition of inosine (a precursor of hypoxanthine) and xanthine led to 1.4- and 1.7-fold increase in butanol production in furfural-challenged cultures of C. beijerinckii. Taken together, our results provide a purine salvage-based rationale for the unanticipated effect of allopurinol in improving furfural tolerance of the ABE-fermenting C. beijerinckii.








Similar content being viewed by others
References
Almeida JRM, Röder A, Modig T, Laadan B, Lidén G, Gorwa-Grauslund MF (2008) NADH- vs NADPH-coupled reduction of 5-hydroxymethyl furfural (HMF) and its implications on product distribution in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 78:939–945
Almeida JRM, Bertilsson M, Gorwa-Grauslund MF, Gorsich S, Lidén G (2009) Metabolic effects of furaldehydes and impacts on biotechnological processes. Appl Microbiol Biotechnol 82:625–638
Alriksson B, Hovarth IS, Johnson LJ (2010) Overexpression of Saccharomyces cerevisiae transcription factor and multidrug resistance genes conveys enhanced resistance to lignocellulose-derived fermentation inhibitors. Process Biochem 45:264–271
Arnér ESJ, Holmgren A (2000) Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267:6102–6109
Ask M, Maurizio B, Mapelli V, Olsson L (2013) The influence of HMF and furfural on the redox-balance and energy-state of xylose and energy-state of xylose-utilizing Saccharomyces cerevisiae. Biotechnol Biofuels 6:22–34
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Christiansen LC, Schou S, Nygaard P, Saxlid HH (1997) Xanthine metabolism in Bacillus subtilis: characterization of the xpt-pbutX operon and evidence of purine- and nitrogen-controlled expression of genes involved in xanthine salvage catabolism. J Bacteriol 179:2540–2550
Corte DE, Stirpe F (1968) The regulation of xanthine oxidase in rat liver: modification of the enzyme activity of rat liver supernatant on storage at -20 °C. Biochem J 108:349–351
Das DK, Engelman RM, Clement R, Otani H, Prasad MR, Rao PS (1987) Role of xanthine oxidase inhibitor as a free radical scavenger: a novel mechanism of action of allopurinol and oxypurinnol in myocardial salvage. Biochem Biophys Res Commun 148:314–319
Dash SS, Gummadi SN (2006) Catabolic pathways and biotechnological applications of microbial caffeine degradation. Biotechnol Lett 28:1993–2002
Edwards NL, Recker D, Airozo D, Fox IH (1981) Enhanced purine salvage during allopurinol therapy: an important pharmacological property in humans. J Lab Clin Med 98:673–683
Engerson TD, McKelvey TG, Rhyne DB, Boggio EB, Snyder JS, Jones HP (1987) Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest 79:1564–1570
Ezeji TC, Groberg M, Qureshi N, Blaschek HP (2003) Continuous production of butanol from starch-based packing peanuts. Appl Biochem Biotechnol 108:375–382
Ezeji TC, Qureshi N, Blaschek HP (2004) Acetone butanol ethanol (ABE) production from concentrated substrate: reduction in substrate inhibition by fed-batch technique and product inhibition by gas stripping. Appl Microbiol Biotechnol 63:653–658
Gapes JR (2000) The economics of acetone-butanol fermentation: theoretical and market considerations. J Mol Microbiol Biotechnol 2:27–32
Kelley WN, Rosenbloom FM, Miller J, Seegmiller JE (1968) An enzymatic basis for variations in response to allopurinol. New Engl J Med 278:287–293
Khan QA, Hadi SM (1993) Effect of furfural on plasmid DNA. Biochem Mol Biol Int 29:1153–1160
Kilstrup M, Hammer K, Jensen PR, Martinussen J (2005) Nucleotide metabolism and its control in lactic acid bacteria. FEMS Microbiol Rev 29:555–590
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26
Koenig K, Andreesen JR (1990) Xanthine dehydrogenase and 2-furoyl-Coenzyme A dehydrogenase from Pseudomonas putida Fu1: two molybdenum-containing dehydrogenases of novel structural composition. J Bacteriol 172:5999–6009
Kolberg M, Strand KR, Graff P, Anderson KK (2004) Structure, function and mechanism of ribonucleotide reductases. Biochim Biophys Acta 1699:1–34
Koopman F, Wierckx N, de Winde JH, Ruijssenaars HJ (2010) Identification and characterization of furfural and 5-(hydroxymethyl)furfural degradation pathways of Cupriavidus basilensis HMF14. Proc Natl Acad Sci U S A 107:4919–4924
López MJ, Nichols NN, Dien BS, Moreno J, Bothast RJ (2004) Isolation of microorganisms for biological detoxification of lignocellulosic hydrolysates. Appl Microbiol Biotechnol 64:125–131
Masip L, Veeravalli K, Georgiou G (2006) The many faces of glutathione in bacteria. Antioxid Redox Signal 8:753–762
Mathews RG (1996) One-carbon metabolism, p 600-611. In: Neidhardt FC, Curtis R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington DC
Miller EN, Jarboe LR, Yomano LP, York SW, Shanmugan KT, Ingram LO (2009) Silencing NADPH-dependent oxidoreductase genes (yqhD and dkgA) in furfural-resistant ethanologenic Escherichia coli. Appl Environ Microbiol 75:4315–4323
Moorhouse PC, Grootveld M, Halliwell B, Quinlan JG, Gutteridge JMC (1987) Allopurinol and oxypurinol are hydroxyl radical scavengers. FEB Lett 213:23–28
Nichols NN, Mertens JA (2008) Identification and transcriptional profiling of Pseudomonas putida genes involved in furoic acid metabolism. FEMS Microbiol Lett 284:82–87
Okuda N, Soneura M, Ninomiya K, Katakura Y, Shioya S (2008) Biological detoxification of waste house wood hydrolysate using Ureibacillus thermosphaericus for bioethanol production. J Biosci Bioeng 106:128–133
Palmqvist E, Almeida JS, Hähn-Hägerdal B (1999) Influence of furfural on anaerobic glycolytic kinetics of Saccharomyces cerevisiae in batch culture. Biotechnol Bioeng 62:447–454
Park S-E, Koo HM, Park YK, Park SM, Park JC, Lee O-K, Park Y-C, Seo J-H (2011) Expression of aldehyde dehydrogenase 6 reduces inhibitory effect of furan derivatives and ethanol production by Saccharomyces cerevisiae. Biores Technol 102:6033–6038
Rouf MA, Lomprey RF (1968) Degradation of uric acid by certain aerobic bacteria. J Bacteriol 96:617–622
Rundles RW, Wyngaarden JB, Hitchings GH, Ellion GB, Silberman HR (1963) Effects of xanthine oxidase inhibitor on thiopurine metabolism, hyperuricemia and gout. Tr Am Phys 76:126–140
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1110
Truglio JJ, Theis K, Leimkhüler S, Rppa R, Rajagopalan KV, Kisker C (2002) Crystal structures of the active and alloxanthine-inhibited forms of xanthine dehydrogenase from Rhodobacter capsulatus. Structure 10:115–125
Ujor V, Agu CV, Gopalan V, Ezeji TC (2014) Glycerol supplementation enhances furfural detoxification by Clostridium beijerinckii during butanol fermentation. Appl Microbiol Biotechnol 98:6511–6521
Wang X, Zhang J, Xin X, Bao J (2012) Furfural degradation by filamentous fungus Amorphotheca seniae ZN1. Chin J Biotechnol 28:1070–1079
Watanabe Y, Ohe T, Tsujisaka Y (1976) Changes in the metabolic pathways of hypoxanthine in Streptomyces. J Gen Appl Microbiol 22:13–23
Waud WR, Rajagopalan KV (1976) Purification and properties of the NAD + -dependent (Type D) and O2-dependent (Type O) forms of rat liver xanthine dehydrogenase. Arch Biochem Biophys 172:354–364
Wierckx N, Koopman F, Ruijssenaars HJ, de Winde JH (2011) Microbial degradation of furanic compounds: biochemistry, genetics and impacts. Appl Microbiol Biotechnol 92:1095–1105
Woolfolk CA (1975) Metabolism of N-methylpurines by a Pseudomonas putida strain isolated by enrichment on caffeine as sole source of carbon and nitrogen. J Bacteriol 123:1088–1106
Woolfolk CA, Woolfolk BS, Whiteley HR (1970) 2-oxypurine dehydrogenase from Micrococcus aerogenes. J Biol Chem 245:3167–3178
Xi H, Schneider BL, Reitzer L (2000) Purine catabolism in Escherichia coli and function of xanthine dehydrogenase in purine salvage. J Bacteriol 182:5332–5341
Yerushalmi L, Volesky B, Leung WK, Neufeld RJ (1983) Variations of solvent yield in acetone-butanol fermentation. Eur J Appl Microbiol Biotechnol 18:279–286
Zdzienicka M, Tudek B, Zielenska M, Szymczyk T (1978) Mutagenic activity of furfural in Salmonella typhimurium TA100. Mutat Res 58:205–209
Zhang Y (2013) Detoxification of lignocellulose-derived microbial inhibitory compounds by Clostridium beijerinckii NCIMB 8052 during acetone-butanol-ethanol fermentation. PhD Dissertation. The Ohio State University
Zhang Y, Ezeji TC (2013) Transcriptional analysis of Clostridium beijerinckii NCIMB 8052 to elucidate the role of furfural stress during acetone butanol fermentation. Biotechnol Biofuels 6:66–82
Zhang Y, Ezeji TC (2014) Elucidating and alleviating impacts of lignocellulose-derived microbial inhibitors on Clostridium beijerinckii during fermentation of Miscanthus giganteus to butanol. J Ind Microbiol Biotechnol 41:1505–1516
Zhang Y, Han B, Ezeji TC (2012) Biotransformation of furfural and 5-hydroxymethyl furfural by Clostridium acetobutylicum ATCC 824 during butanol fermentation. New Biotechnol 29:345–351
Zheng H, Wang X, Yomano LP, Shanmugan KT, Ingram LO (2012) Increase in furfural tolerance in ethanologenic Escherichia coli LY180 by plasmid-based expression of thyA. Appl Environ Microbiol 78:4346–4352
Acknowledgments
Salaries and research support were provided in part by State funds appropriated to the Ohio Plant Biotechnology Consortium by The Ohio State University, Ohio Agricultural Research and Development Center (OARDC), and the Hatch grant (Project No. OHO01333). We are grateful to Dr. Joseph Hogan (Department of Animal Sciences, The Ohio State University & OARDC) for allowing us use his iMark™ multi-well plate reader for NAD+/NADH and NADP+/NADPH assays.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ujor, V., Agu, C.V., Gopalan, V. et al. Allopurinol-mediated lignocellulose-derived microbial inhibitor tolerance by Clostridium beijerinckii during acetone–butanol–ethanol (ABE) fermentation. Appl Microbiol Biotechnol 99, 3729–3740 (2015). https://doi.org/10.1007/s00253-015-6450-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6450-3