Abstract
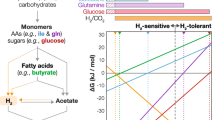
Methanogenesis is central to anaerobic digestion processes. The conversion of propionate as a key intermediate for methanogenesis requires syntrophic interactions between bacterial and archaeal partners. In this study, a series of methanogenic enrichments with propionate as the sole substrate were developed to identify microbial populations specifically involved in syntrophic propionate conversion. These rigorously controlled propionate enrichments exhibited functional stability with consistent propionate conversion and methane production; yet, the methanogenic microbial communities experienced substantial temporal dynamics, which has important implications on the understanding of mechanisms involved in microbial community assembly in anaerobic digestion. Syntrophobacter was identified as the most abundant and consistent bacterial partner in syntrophic propionate conversion regardless of the origin of the source culture, the concentration of propionate, or the temporal dynamics of the culture. In contrast, the methanogen partners involved in syntrophic propionate conversion lacked consistency, as the dominant methanogens varied as a function of process condition and temporal dynamics. Methanoculleus populations were specifically enriched as the syntrophic partner at inhibitory levels of propionate, likely due to the ability to function under unfavorable environmental conditions. Syntrophic propionate conversion was carried out exclusively via transformation of propionate into acetate and hydrogen in enrichments established in this study. Microbial populations highly tolerant of elevated propionate, represented by Syntrophobacter and Methanoculleus, are of great significance in understanding methanogenic activities during process perturbations when propionate accumulation is frequently encountered. Key points • Syntrophobacter was the most consistent bacterial partner in propionate metabolism. • Diverse hydrogenotrophic methanogen populations could serve as syntrophic partners. • Methanoculleus emerged as a methanogen partner tolerant of elevated propionate.


source culture in replicates. a Community composition at the domain level; b archaeal populations; and c bacterial populations. Microbial populations in b and c are shown at the genus level. Only populations with relative abundance greater than 1% are shown





source cultures including dilute diary manure (D), beef cattle manure (C), excess sludge from a secondary municipal wastewater treatment facility (S), digestate from a bench-scale anaerobic bioreactor developed with sucrose as the sole substrate (W), and digestate from a bench-scale anaerobic digester developed with dilute diary manure as the sole substrate (L). Shown are genera with average relative abundance > 1%
Similar content being viewed by others
Data availability
Sequence data is available at the Sequence Read Archive (SRA) of GenBank (https://www.ncbi.nlm.nih.gov/sra) with accession numbers SAMN12719674–SAMN12719684. Other data and material for this article are available upon request.
References
Acharya BK, Pathak H, Mohana S, Shouche Y, Singh V, Madamwar D (2011) Kinetic modelling and microbial community assessment of anaerobic biphasic fixed film bioreactor treating distillery spent wash. Water Res 45(14):4248–4259
Angenent LT, Sung S, Raskin L (2002) Methanogenic population dynamics during startup of a full-scale anaerobic sequencing batch reactor treating swine waste. Water Res 36(18):4648–4654
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, D.C
Barret M, Gagnon N, Kalmokoff ML, Topp E, Verastegui Y, Brooks SPJ, Matias F, Neufeld JD, Talbot G (2013) Identification of Methanoculleus spp. as active methanogens during anoxic incubations of swine manure storage tank samples. Appl Environ Micr 79(2):424–433
Barret M, Gagnon N, Morissette B, Topp E, Kalmokoff M, Brooks SPJ, Matias F, Masse DI, Masse L, Talbot G (2012) Methanoculleus spp. as a biomarker of methanogenic activity in swine manure storage tanks. FEMS Microbiol Ecol 80(2):427–440
Boone DR, Bryant MP (1980) Propionate-degrading bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from methanogenic ecosystems. Appl Environ Microbiol 40(3):626–632
Botello Suárez WA, Da Silva VJ, Duda RM, Giachetto PF, Cintra LC, Tiraboschi Ferro MI, de Oliveira RA (2018) Predominance of syntrophic bacteria, Methanosaeta and Methanoculleus in a two-stage up-flow anaerobic sludge blanket reactor treating coffee processing wastewater at high organic loading rate. Bioresour Technol 268:158–168
Botsch KC, Conrad R (2011) Fractionation of stable carbon isotopes during anaerobic production and degradation of propionate in defined microbial cultures. Org Geochem 42(3):289–295
Capson-Tojo G, Ruiz D, Rouez M, Crest M, Steyer JP, Bernet N, Delgenes JP, Escudie R (2017) Accumulation of propionic acid during consecutive batch anaerobic digestion of commercial food waste. Bioresour Technol 245:724–733
Chen S, He Q (2016) Distinctive non-methanogen archaeal populations in anaerobic digestion. Appl Microbiol Biotechnol 100(1):419–430
Chen S, Liu X, Dong X (2005) Syntrophobacter sulfatireducens sp. nov., a novel syntrophic, propionate-oxidizing bacterium isolated from UASB reactors. Int J Syst Evol Microbiol 55(Pt 3):1319–1324
Chen S, Zamudio Canas EM, Zhang Y, Zhu Z, He Q (2012) Impact of substrate overloading on archaeal populations in anaerobic digestion of animal waste. J Appl Microbiol 113:1371–1379
Chen YT, Zeng Y, Wang HZ, Zheng D, Kamagata Y, Narihiro T, Nobu MK, Tang YQ (2020) Different interspecies electron transfer patterns during mesophilic and thermophilic syntrophic propionate degradation in chemostats. Environ Microbiol 80:120–132
De Bok FAM, Hagedoorn PL, Silva PJ, Hagen WR, Schiltz E, Fritsche K, Stams AJM (2003) Two W-containing formate dehydrogenases (CO2-reductases) involved in syntrophic propionate oxidation by Syntrophobacter fumaroxidans. Eur J Biochem 270(11):2476–2485
De Bok FAM, Stams AJM, Dijkema C, Boone DR (2001) Pathway of propionate oxidation by a syntrophic culture of Methanospirillum hungatei. Appl Environ Microbiol 67(4):1800–1804
Demirer GN, Speece RE (1998) Anaerobic biotransformation of four 3-carbon compounds (acrolein, acrylic acid, allyl alcohol and n-propanol) in UASB reactors. Water Res 32(3):747–759
Dolfing J (2013) Syntrophic propionate oxidation via butyrate: a novel window of opportunity under methanogenic conditions. Appl Environ Microbiol 79(14):4515–4516
Dong X, Plugge CM, Stams AJM (1994) Anaerobic degradation of propionate by a mesophilic acetogenic bacterium in coculture and triculture with different methanogens. Appl Environ Microbiol 60(8):2834–2838
Enzmann F, Mayer F, Rother M, Holtmann D (2018) Methanogens: biochemical background and biotechnological applications. AMB Express 8(1):1
Fontana A, Patrone V, Puglisi E, Morelli L, Bassi D, Garuti M, Rossi L, Cappa F (2016) Effects of geographic area, feedstock, temperature, and operating time on microbial communities of six full-scale biogas plants. Bioresour Technol 218:980–990
Gallert C, Winter J (2008) Propionic acid accumulation and degradation during restart of a full-scale anaerobic biowaste digester. Bioresour Technol 99(1):170–178
Han Y, Green H, Tao W (2020) Reversibility of propionic acid inhibition to anaerobic digestion: inhibition kinetics and microbial mechanism. Chemosphere 255(126840):0045–6535
Houwen FP, Plokker J, Stams AJM, Zehnder AJB (1990) Enzymatic evidence for involvement of the methylmalonyl-CoA pathway in propionate oxidation by Syntrophobacter wolinii. Arch Microbiol 155:52–55
Imachi H, Sakai S, Ohashi A, Harada H, Hanada S, Kamagata Y, Sekiguchi Y (2007) Pelotomaculum propionicicum sp. nov., an anaerobic, mesophilic, obligately syntrophic, propionate-oxidizing bacterium. Int J Syst Evol Microbiol 57(pt 7):1487–1492
Jannat MAH, Lee J, Shin SG, Hwang S (2021) Long-term enrichment of anaerobic propionate-oxidizing consortia: syntrophic culture development and growth optimization. J Hazard Mater 401:123230
Lavania M, Cheema S, Sarma PM, Ganapathi R, Lal B (2014) Methanogenic potential of a thermophilic consortium enriched from coal mine. Int Biodeterior Biodegrad 93:177–185
Li C, Moertelmaier C, Winter J, Gallert C (2015) Microbial community shifts during biogas production from biowaste and/or propionate. Bioengineering (basel, Switzerland) 2(1):35–53
Li J, Ban Q, Zhang L, Jha A (2012) Syntrophic propionate degradation in anaerobic digestion: a review. Int J Agric Biol 14(5):843–850
Li Y, Sun Y, Li L, Yuan Z (2018) Acclimation of acid-tolerant methanogenic propionate-utilizing culture and microbial community dissecting. Bioresour Technol 250:117–123
Li Y, Sun Y, Yang G, Hu K, Lv P, Li L (2017a) Vertical distribution of microbial community and metabolic pathway in a methanogenic propionate degradation bioreactor. Bioresour Technol 245:1022–1029
Li Y, Zhang Y, Sun Y, Wu S, Kong X, Yuan Z, Dong R (2017b) The performance efficiency of bioaugmentation to prevent anaerobic digestion failure from ammonia and propionate inhibition. Bioresour Technol 231:94–100
Liu RR, Tian Q, Yang B, Chen JH (2010) Hybrid anaerobic baffled reactor for treatment of desizing wastewater. Int J Environ Sci Technol 7:111–118
Liu Y, Balkwill DL, Aldrich HC, Drake GR, Boone DR (1999) Characterization of the anaerobic propionate-degrading syntrophs Smithella propionica gen. nov., sp. nov. and Syntrophobacter wolinii. Int J Syst Evol Microbiol 49(Pt 2):545–556
McCarty PL, Smith DP (1986) Anaerobic wastewater treatment. Environ Sci Technol 20(12):1200–1206
McMahon KD, Zheng D, Stams AJ, Mackie RI, Raskin L (2004) Microbial population dynamics during start-up and overload conditions of anaerobic digesters treating municipal solid waste and sewage sludge. Biotechnol Bioeng 87(7):823–834
Muller N, Worm P, Schink B, Stams AJ, Plugge CM (2010) Syntrophic butyrate and propionate oxidation processes: from genomes to reaction mechanisms. Environ Microbiol Rep 2(4):489–499
Narihiro T, Nobu MK, Kim NK, Kamagata Y, Liu WT (2015) The nexus of syntrophy-associated microbiota in anaerobic digestion revealed by long-term enrichment and community survey. Environ Microbiol 17:1707–1720
Nobu MK, Narihiro T, Rinke C, Kamagata Y, Tringe SG, Woyke T, Liu WT (2015) Microbial dark matter ecogenomics reveals complex synergistic networks in a methanogenic bioreactor. ISME J 9(8):1710–1722
Puengrang P, Suraraksa B, Prommeenate P, Boonapatcharoen N, Cheevadhanarak S, Tanticharoen M, Kusonmano K (2020) Diverse microbial community profiles of propionate-degrading cultures derived from different sludge sources of anaerobic wastewater treatment plants. Microorganisms 8:277
Pullammanappallil PC, Chynoweth DP, Lyberatos G, Svoronos SA (2001) Stable performance of anaerobic digestion in the presence of a high concentration of propionic acid. BioresourTechnol 78(2):165–169
Rajendran K, Mahapatra D, Venkatraman AV, Muthuswamy S, Pugazhendhi A (2020) Advancing anaerobic digestion through two-stage processes: current developments and future trends. Renew Sust Energ Rev 123:109746
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev 61(2):262–280
Tale VP, Maki JS, Struble CA, Zitomer DH (2011) Methanogen community structure-activity relationship and bioaugmentation of overloaded anaerobic digesters. Water Res 45(16):5249–5256
Venkiteshwaran K, Bocher B, Maki J, Zitomer D (2016) Relating anaerobic digestion microbial community and process function. Microbiol Insights 8(Suppl 2):37–44
Wang HZ, Yan YC, Gou M, Yi Y, Xia ZY, Nobu MK, Narihiro T, Tang YQ (2019) Response of propionate-degrading methanogenic microbial communities to inhibitory conditions. Appl Biochem Biotechnol 189:233–248
Wang Y, He Q (2019) Microbial interactions in anaerobic wastewater treatment. In: Maurice PA (ed) Encyclopedia of water: science, technology, and society. John Wiley & Sons, Inc., Hoboken, New Jersey, pp 2231–2238. https://doi.org/10.1002/9781119300762.wsts0196
Ye M, Liu JY, Ma CN, Li YY, Zou LP, Qian GG, Xu P (2018) Improving the stability and efficiency of anaerobic digestion of food waste using additives: a critical review. J Clean Prod 192:316–326
Yilmaz P, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, Schweer T, Peplies J, Ludwig W, Glochner FO (2014) The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res 42(D1):D643–D648
Zhang Y, Zamudio Cañas E, Zhu Z, Linville J, Chen S, He Q (2011) Robustness of archaeal populations in anaerobic co-digestion of dairy and poultry wastes. Bioresour Technol 102(2):779–785
Funding
This work was supported in part by U.S. National Science Foundation (NSF) award 2025339. LC was partly supported by the Department of Civil and Environmental Engineering, University of Tennessee, Knoxville.
Author information
Authors and Affiliations
Contributions
LC and QH conceived and designed research. LC conducted experiments. LC analyzed data. LC, QH, and CDC wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Disclaimer
Any opinions, findings, recommendations, and conclusions in this paper are those of the authors, and do not necessarily reflect the views of NSF and University of Tennessee, Knoxville.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, L., Cox, C.D. & He, Q. Patterns of syntrophic interactions in methanogenic conversion of propionate. Appl Microbiol Biotechnol 105, 8937–8949 (2021). https://doi.org/10.1007/s00253-021-11645-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11645-9