Abstract
Purpose
The combination of positron emission tomography (PET) and magnetic resonance (MR) tomography in a single device is anticipated to be the next step following PET/CT for future molecular imaging application. Compared to CT, the main advantages of MR are versatile soft tissue contrast and its capability to acquire functional information without ionizing radiation. However, MR is not capable of measuring a physical quantity that would allow a direct derivation of the attenuation values for high-energy photons.
Methods
To overcome this problem, we propose a fully automated approach that uses a dedicated T1-weighted MR sequence in combination with a customized image processing technique to derive attenuation maps for whole-body PET. The algorithm automatically identifies the outer contour of the body and the lungs using region-growing techniques in combination with an intensity analysis for automatic threshold estimation. No user interaction is required to generate the attenuation map.
Results
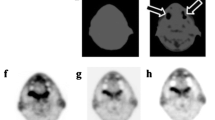
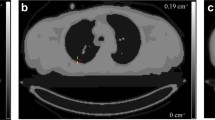
The accuracy of the proposed MR-based attenuation correction (AC) approach was evaluated in a clinical study using whole-body PET/CT and MR images of the same patients (n = 15). The segmentation of the body and lung contour (L-R directions) was evaluated via a four-point scale in comparison to the original MR image (mean values >3.8). PET images were reconstructed using elastically registered MR-based and CT-based (segmented and non-segmented) attenuation maps. The MR-based AC showed similar behaviour as CT-based AC and similar accuracy as offered by segmented CT-based AC. Standardized uptake value (SUV) comparisons with reference to CT-based AC using predefined attenuation coefficients showed the largest difference for bone lesions (mean value ± standard variation of SUVmax: −3.0% ± 3.9% for MR; −6.5% ± 4.1% for segmented CT). A blind comparison of PET images corrected with segmented MR-based, CT-based and segmented CT-based AC afforded identical lesion detectability, but slight differences in image quality were found.
Conclusion
Our MR‐based attenuation correction method offers similar correction accuracy as offered by segmented CT. According to the specialists involved in the blind study, these differences do not affect the diagnostic value of the PET images.















Similar content being viewed by others
References
Kops ER, Herzog H. Alternative methods for attenuation correction for PET images in MR-PET scanners. IEEE Nucl Sci Symp Conf Rec 2007;6:4327–30.
Catana A, et al. Is accurate bone segmentation required for MR-based PET attenuation correction? Proc Intl Soc Mag Reson Med 2009;17:593.
Beyer T, Weigert M, Quick HH, Pietrzyk U, Vogt F, Palm C, et al. MR-based attenuation correction for torso-PET/MR imaging: pitfalls in mapping MR to CT data. Eur J Nucl Med Mol Imaging 2008;35:1142–6.
van der Kouwe AJ, et al. Challenges for MR-based attenuation correction in PET imaging of the head. Proc Intl Soc Mag Reson Med 2009;17:2810.
Marshall HR, et al. Use of multi-spectral MR data to generate an attenuation map for application to PET/MR hybrid imaging. Proc Intl Soc Mag Reson Med 2009;17:4698.
Hofmann M, et al. MR-based attenuation correction for PET/MR. Proc Intl Soc Mag Reson Med 2009;17:260.
Martinez-Möller A, Souvatzoglou M, Delso G, Bundschuh RA, Chefd’hotel C, Ziegler SI, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med 2009;50:520–6.
Hofmann M, Steinke F, Scheel V, Charpiat G, Farquhar J, Aschoff P, et al. MRI-based attenuation correction for PET/MR: a novel approach combining pattern recognition and atlas registration. J Nucl Med 2008;49:1875–83.
Hofmann M, Pichler B, Schölkopf B, Beyer T. Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques. Eur J Nucl Med Mol Imaging 2009;36(Suppl 1):S93–104. doi:10.1007/s00259-008-1007-7.
Bai C, Shao L, Da Silva A, Zhao Z. A generalized model for the conversion from CT numbers to linear attenuation coefficients. IEEE Trans Nucl Sci 2003;50:1510–5.
Zaidi H. Is MR-guided attenuation correction a viable option for dual-modality PET/MR imaging? Radiology 2007;244:639–42.
Zaidi H, Montandon ML, Slosman DO. Magnetic resonance imaging-guided attenuation and scatter corrections in three-dimensional brain positron emission tomography. Med Phys 2003;30(5):937–48.
Salomon A, Schulz V, Brinks R, Schweizer B, Goedicke A. Iterative generation of attenuation maps in TOF-PET/MR using consistency conditions. SNM’s 56th Annual Meeting, 13–17 June 2009.
Wu TH, Huang YH, Lee JJ, Wang SY, Su CT, Chen LK, et al. Radiation exposure during transmission measurements: comparison between CT- and germanium-based techniques with a current PET scanner. Eur J Nucl Med Mol Imaging 2004;31:38–43.
Zaidi H. Is radionuclide transmission scanning obsolete for dual-modality PET/CT systems? Eur J Nucl Med Mol Imaging 2007;34:815–8.
Hu Z, Ojha N, Renisch S, Schulz V, Torres I, Buhl A, et al. MR-based attenuation correction for a whole-body sequential PET/MR system. IEEE Nucl Sci Symp Conf Rec 2009;M11–6.
Sensakovic WF, Armato SG. Magnetic resonance imaging of the lung: automated segmentation methods. Methods of cancer diagnosis, therapy, and prognosis, Vol 2. Netherlands: Springer.
Valk PE, Bailey DL, Townsend DW, Maisey MN. Positron emission tomography: basic science and clinical practice. London: Springer; 2004. 3rd printing.
Wiemker R, Pekar V. Fast computation of isosurface contour spectra for volume visualization. Proc Computer Assisted Radiology and Surgery 2001;1230:389–94.
Rueckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging 1999;18:712–21.
Wang W, Hu Z, Gualtieri EE, Parma MJ, Walsh ES, Sebok D, et al. Systematic and distributed time-of-flight list mode PET reconstruction. IEEE Nucl Sci Symp Conf Rec 2006;3:1715–22.
Verschakelen JA, Van fraeyenhoven L, Laureys G, Demedts M, Baert AL. Differences in CT density between dependent and nondependent portions of the lung: influence of lung volume. AJR Am J Roentgenol 1993;161:713–7.
Tawhai MH, Nash MP, Lin CL, Hoffman EA. Supine and prone differences in regional lung density and pleural pressure gradients in the human lung with constant shape. J Appl Physiol 2009;107:912–20.
Corder GW, Foreman DI. Nonparametric statistics for non-statisticians: a step-by-step approach. New Jersey: Wiley; 2009.
Keereman V, et al. Estimation of attenuation maps from UTE derived R2 image. Proc Intl Soc Mag Reson Med 2009;17:2774.
Robson MD, Bydder GM. Clinical ultrashort echo time imaging of bone and other connective tissues. NMR Biomed 2006;19:765–80.
Ma J, Costelloe CM, Madewell JE, Hortobagyi GN, Green MC, Cao G, et al. Fast dixon-based multisequence and multiplanar MRI for whole-body detection of cancer metastases. J Magn Reson Imaging 2009;29(5):1154–62.
Madsen MT. PET attenuation correction using mean attenuation coefficients: a simulation study. IEEE Trans Nucl Sci 1999;46(6):2172–6.
Beyer T, Bockisch A, Kühl H, Martinez MJ. Whole-body 18F-FDG PET/CT in the presence of truncation artifacts. J Nucl Med 2006;47(1):91–9.
Goerres GW, Ziegler SI, Burger C, Berthold T, Von Schulthess GK, Buck A. Artifacts at PET and PET/CT caused by metallic hip prosthetic material. Radiology 2003;226:577–84.
Berthelsen AK, Holm S, Loft A, Klausen TL, Andersen F, Højgaard L. PET/CT with intravenous contrast can be used for PET attenuation correction in cancer patients. Eur J Nucl Med Mol Imaging 2005;32:1167–75.
Acknowledgment
This work was supported by the EU FP7 project HYPERImage (grant agreement 201651).
Conflicts of interest
The first seven authors are employees of Philips.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schulz, V., Torres-Espallardo, I., Renisch, S. et al. Automatic, three-segment, MR-based attenuation correction for whole-body PET/MR data. Eur J Nucl Med Mol Imaging 38, 138–152 (2011). https://doi.org/10.1007/s00259-010-1603-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1603-1